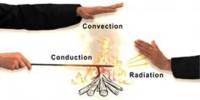
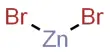
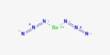
Basic objective of this lecture is to focus on Relative mass formula, atomic mass, and empirical formula. The relative formula mass of a compound is blatantly the relative atomic masses of all the elements in the compound added together. The mass of an isotopic element relative to Carbon-12. Example: chlorine occurs in isotope forms Cl-35 (75.5%) and Cl-37 (24.5%). Here also focus on how to calculating the mass of a product.
Relative Mass Formula, Atomic Mass and Empirical Formula