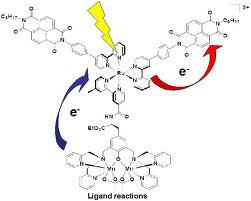
Basic purpose of this article is to Discuss on Electron Transfer. In the electron transfer reaction, a feature undergoing oxidation loses electrons, whereas a feature gaining electrons undergoes decline. In the aluminum‐oxygen case in point, the aluminum was oxidized, and also the oxygen was reduced because every electron transfer problem involves simultaneous oxidation in addition to reduction. These reactions are generally called redox reactions.
Discuss on Electron Transfer