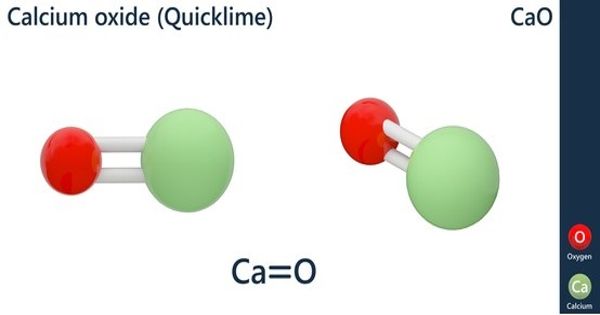
Calcium oxide (CaO) is an alkaline substance that has been in use since the medieval age. It is commonly known as quicklime or burnt lime is a widely used chemical compound. It is a member of the class of calcium oxides of calcium and oxygen in a 1:1 ratio. It is a white, caustic, alkaline, crystalline solid at room temperature. It is believed that quicklime is one of the oldest chemicals known to the human race. The broadly used term “lime” connotes calcium-containing inorganic materials, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminum, and iron predominate. It can also be referred to as burnt lime or lime. It has a role as a fertilizer.
By contrast, quicklime specifically applies to the single chemical compound calcium oxide. CaO is one of the most favorable heterogeneous base catalysts due to their relatively high basic sites, nontoxic, low solubility in methanol, and can be prepared from cheap resources like limestone and calcium hydroxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime.

Properties
Calcium oxide appears as an odorless, white or gray-white solid in the form of hard lumps. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. It has a moderate effect on colour, except in large amounts when it may have a bleaching effect on iron oxide.
- Molecular Weight: 56.08
- Appearance: White Powder
- Melting Point: 2,572° C (4,662° F)
- Boiling Point: 2850 °C (5162 °F)
- Density: 3.3 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 55.9575 g/mol
- Monoisotopic Mass: 55.957506 Da
Preparation
Calcium oxide has a medium viscosity and a high surface tension, plus a high to intermediate expansion and contraction rate. It is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln.
Calcium oxide can be produced by the thermal decomposition of materials like limestone or seashells that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above 825°C (1,517°F), a process called calcination or lime-burning, to liberate a molecule of carbon dioxide (CO2), leaving quicklime. The process that is used to prepare burnt lime is known as calcination. It is a process that starts with thermally decomposing the reactants at high temperatures but ensuring that the temperature is kept well below the melting point.
CaCO3(s) → CaO(s) + CO2(g)
The quicklime is not stable and, when cooled, will spontaneously react with CO2 from the air until, after enough time, it will be completely converted back to calcium carbonate unless slaked with water to set as lime plaster or lime mortar.
Annual worldwide production of quicklime is around 283 million tonnes. China is by far the world’s largest producer, with a total of around 170 million tonnes per year.
Uses
- It is used in insecticides and fertilizers.
- It is extensively used for medicinal purposes and insecticides.
- It finds its application in the manufacturing of cement, paper, and high-grade steel.
- Lime is used as a reagent in laboratories for dehydration, precipitation reaction, etc.
- It is the cheapest alkali available which is an important ingredient in the manufacturing of caustic soda.
Information Source: