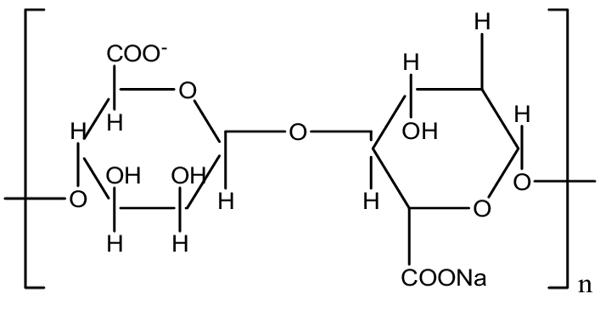
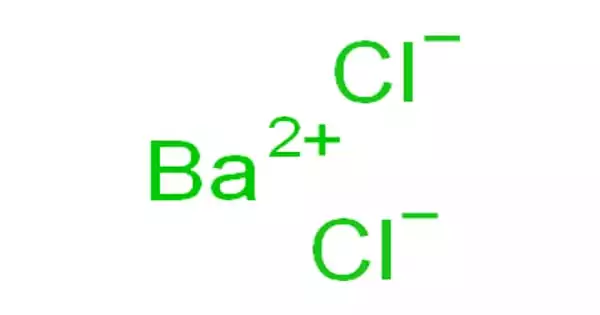Calcium alginate is the insoluble form of alginate with a high swelling capacity. It is a water-insoluble, gelatinous, cream-colored substance that can be created through the addition of aqueous calcium chloride to aqueous sodium alginate. It can be woven to produce pliable patches or ribbons for filling cavities or tunneling wounds. Calcium alginate is also used for the entrapment of enzymes and forming artificial seeds in plant tissue culture.
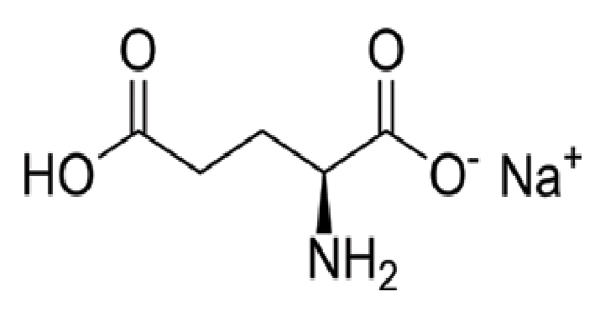
“Alginate” is usually the salts of alginic acid, but it can also refer to derivatives of alginic acid and alginic acid itself; in some publications, the term “algin” is used instead of alginate. Alginate is a general term for a family of polysaccharides produced by seaweed, brown algae, and bacteria. Alginate is present in the cell walls of brown algae, as the calcium, magnesium, and sodium salts of alginic acid.
Preparation
- Extraction of alginate
To extract the alginate, the seaweed is broken into pieces and stirred with a hot solution of an alkali, usually sodium carbonate. Over a period of about two hours, the alginate dissolves as sodium alginate to give a very thick slurry. This slurry also contains the part of the seaweed that does not dissolve, mainly cellulose. This insoluble residue must be removed from the solution. The solution is too thick (viscous) to be filtered and must be diluted with a very large quantity of water. After dilution, the solution is forced through a filter cloth in a filter press. However, the pieces of undissolved residue are very fine and can quickly clog the filter cloth. Therefore, before filtration is started, a filter aid, such as diatomaceous earth, must be added; this holds most of the fine particles away from the surface of the filter cloth and facilitates filtration.
- Preparation of calcium alginate from sodium alginate
The extent of swelling is directly related to the calcium/sodium ratio. Calcium alginate can be produced from a sodium alginate solution by the addition of a calcium salt such as calcium chloride. The sodium alginate donates water molecules out of its initial network, due to the greater tendency of sodium ions to bond with G blocks, which leads to a more dense structure. This forms insoluble calcium alginate salt which precipitates out of solution. The calcium alginate may then be redissolved in various sodium carbonate solutions to produce alginate products containing specific ratios of sodium to calcium. Therefore, sodium ions releasing from alginate can be suggested as an effective water delivery pump for moisturizing the wound environment, particularly in necrotic tissues or dry wounds.
Uses
Uses of calcium alginate are:
- these are used for moderately exudative ulcers and are excellent for tunneling and undermining areas.
- in plant tissue culture to produce insoluble artificial seeds
- for immobilizing enzymes by entrapment
- to produce an edible substance
- incorporated into wound dressings (alginate dressings) as a hemostatic
- as a alginate hydrogel, can be used for a controlled-release drug delivery system
Informatuon Source: