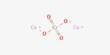
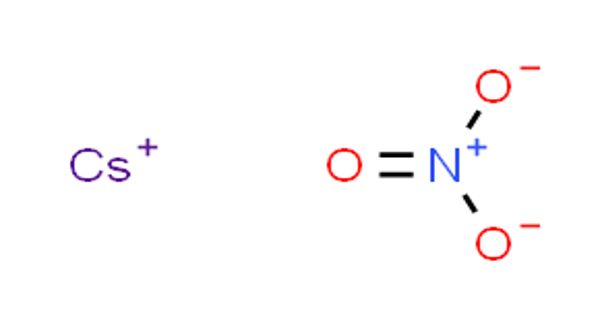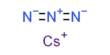Caesium nitrate or cesium nitrate is a salt with the chemical formula CsNO3. It is a colorless, glittering crystalline solid. An alkali metal nitrate is used in pyrotechnic compositions, as a colorant and an oxidizer, e.g. in decoys and illumination flares. It is soluble in water. The caesium emissions are chiefly due to two powerful spectral lines at 852.113 nm and 894.347 nm. It is a strong oxidizing material and may burst into flames in contact with organic materials.
Properties
Cesium nitrate is a colorless, glittering crystalline solid. It is soluble in water. It is a strong oxidizing material and may burst into flames on contact with organic materials. It is silvery gold, soft, and ductile. It is the most electropositive and most alkaline element.
- Molecular Weight: 194.91
- Appearance: White
- Melting Point: N/A
- Boiling Point: N/A
- Density: 3.685 g/cm3
- Solubility in H2O: N/A
- Exact Mass: 194.893
- Monoisotopic Mass: 194.893

Reaction
Caesium nitrate reacts explosively with cold water, and reacts with ice at temperatures above -116°C. Cesium hydroxide is a strong base and attacks glass. Cesium reacts with the halogens to form a fluoride, chloride, bromide, and iodide. Cesium metal oxidized rapidly when exposed to the air and can form the dangerous superoxide on its surface.
As with other alkali metal nitrates, caesium nitrate decomposes on gentle heating to give caesium nitrite:
2CsNO3 → 2CsNO2 + O2
Caesium also forms two unusual acid nitrates, which can be described as CsNO3·HNO3 and CsNO3·2HNO3 (melting points 100 °C and 36–38°C respectively).
Uses
Caesium nitrate prisms are used in infrared spectroscopy, in x-ray phosphors, and in scintillation counters. Cesium salts are used to strenght various types of glass. The chloride is used in photoelectric cells, in optical instruments, and in increasing the sensitivity of electron tubes. It is also used in making optical glasses and lenses.
Information Source: