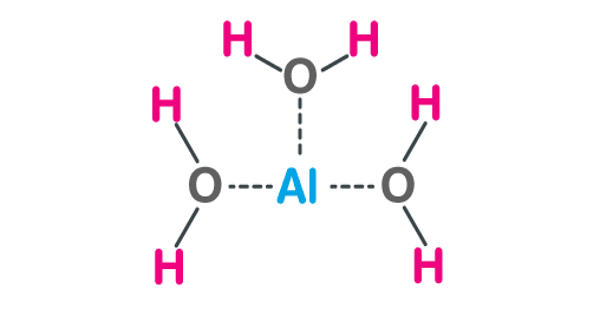
Aluminum Hydroxide, Al(OH)3, is an inorganic compound containing aluminum. It is found in nature as the mineral gibbsite (also known as hydrargillite) and it’s three much rarer polymorphs: bayerite, doyleite, and nordstrandite. It is used in various immunologic preparations to improve immunogenicity, aluminum hydroxide adjuvant consists of aluminum hydroxide gel in a saline solution.
Aluminium hydroxide is amphoteric in nature, i.e., it has both basic and acidic properties. It is a naturally occurring mineral. It is an antacid. Closely related are aluminium oxide hydroxide, AlO(OH), and aluminium oxide or alumina (Al2O3), the latter of which is also amphoteric. These compounds together are the major components of the aluminium ore bauxite. In vaccines, this agent binds to the protein conjugate, resulting in improved antigen processing by the immune system.
Properties
Aluminium hydroxide is amphoteric. In acid, it acts as a Brønsted–Lowry base. It neutralizes the acid, yielding a salt:
3 HCl + Al(OH)3 → AlCl3 + 3 H2O
In bases, it acts as a Lewis acid by binding hydroxide ions:
Al(OH)3 + OH– → Al(OH)4–

Production
Virtually all the aluminium hydroxide used commercially is manufactured by the Bayer process which involves dissolving bauxite in sodium hydroxide at temperatures up to 270 °C (518 °F). The formation of defects in the initial aluminium hydroxide can be initiated by different means: by mechanical activation of aluminium hydroxide, by its thermochemical treatment. The waste solid, bauxite tailings, is removed and aluminium hydroxide is precipitated from the remaining solution of sodium aluminate. This aluminium hydroxide can be converted to aluminium oxide or alumina by calcination. The structural relations between the many crystalline forms of aluminium oxide and hydroxide are exceedingly complex but they are of exceptional scientific interest and immense technological importance.
The residue or bauxite tailings, which is mostly iron oxide, is highly caustic due to residual sodium hydroxide. It was historically stored in lagoons; this led to the Ajka alumina plant accident in 2010 in Hungary, where a dam bursting led to the drowning of nine people. An additional 122 sought treatment for chemical burns. The mud contaminated 40 square kilometres (15 sq mi) of land and reached the Danube. While the mud was considered non-toxic due to low levels of heavy metals, the associated slurry had a pH of 13.
Uses
Aluminum hydroxide is an inorganic salt used as an antacid. It is a basic compound that acts by neutralizing hydrochloric acid in gastric secretions. Subsequent increases in pH may inhibit the action of pepsin. It is used to treat heartburn, upset stomach, sour stomach, or acid indigestion. It is also used to reduce phosphate levels in people with certain kidney conditions.
Information Source: