Researchers from Tokyo Tech have finally identified the redox control mechanism that enables effective ATP synthesis in photosynthetic organisms under a range of light conditions.
Scientists looked into the enzyme that is in charge of this mechanism and discovered how the amino acid sequences in the enzyme control the amount of ATP produced. Their findings offer important new understanding of photosynthesis and the capacity for metabolic adaptation.
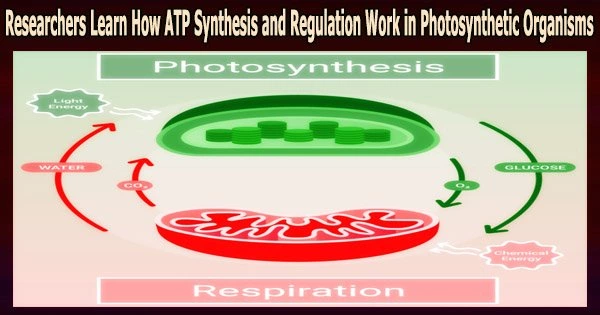
An enzyme called “chloroplast ATP synthase” (CFoCF1) produces ATP, the compound essential for the functioning of photosynthetic organisms such as plants and algae.
The enzyme uses a redox regulatory mechanism that modifies the ATP synthesis activity in response to changes in the redox state of cysteine (Cys) residues, which exist as dithiols under reducing (light) conditions but forms a disulfide bond under oxidizing (dark) conditions. This mechanism allows the enzyme to regulate ATP production under a range of light conditions. However, this mechanism has not been fully understood so far.
Now, in a study published in the Proceedings of the National Academy of Sciences, a team of researchers from Japan, led by Prof. Toru Hisabori from Tokyo Institute of Technology (Tokyo Tech), has uncovered the role of the amino acid sequences present in CFoCF1, revealing how the enzyme regulates ATP production in photosynthetic organisms.
The redox regulation of ATP synthesis is accomplished by a cooperative interaction between two γ subunit domains of CFoCF1 unique to photosynthetic organisms. We propose that it results from the interaction of the β-hairpin and the redox loop with the catalytic site.
Professor Toru Hisabori
The scientists produced the enzyme in Chlamydomonas reinhardtii, a unicellular green alga, to better understand how the conformation of the amino acids in CFoCF1 contributes to the redox control mechanism.
“By leveraging the powerful genetics of Chlamydomonas reinhardtii as a model organism for photosynthesis, we conducted a comprehensive biochemical analysis of the CFoCF1 molecule,” explains Prof. Hisabori.
With the alga as the host organism, the team introduced plasmids (extrachromosomal DNA molecule that can replicate independently) that encoded the F1 component of the CFoCF1 protein, namely the part of the enzyme containing catalytic sites for ATP synthesis. They additionally introduced mutated versions of the gene to change the amino acid sequences of the protein, specifically targeting the DDE motif (a cluster of negatively charged amino acids), the redox loop, and the β-hairpin domain.
They then purified CFoCF1, generating five different variations of it that included a wild-type strain with no changes to the amino acid sequence and four mutant strains: one with the DDE motif replaced with neutral amino acids, Asn-Asn-Gln, one without the β-hairpin domain, one without the redox loop, and one lacking both the redox loop and the β-hairpin domain.
The researchers discovered that the wild-type enzyme and the mutant enzyme with alterations to the DDE motif functioned normally (showed high activity when reduced and low activity when oxidized) after measuring the ATP synthesis activity of these mutants under reducing (simulating the light conditions) and oxidizing (simulating the dark conditions).
However, the enzyme complexes without the redox loop or the β-hairpin domain did not show the redox-response, indicating that both regions were involved in the redox regulation mechanism.
The researchers suggested that under dark conditions, the disulfide bond between the Cys residues makes the redox loop rigid and weakens the interaction between the redox loop and the β-hairpin. This causes the β-hairpin to remain stuck within a cavity in the protein.
The redox loop, on the other hand, regains its flexibility and pulls the β-hairpin out of the cavity when the disulfide bond is decreased in the presence of light, allowing it to participate in the ATP production activity.
“The redox regulation of ATP synthesis is accomplished by a cooperative interaction between two γ subunit domains of CFoCF1 unique to photosynthetic organisms,” says Prof. Hisabori. “We propose that it results from the interaction of the β-hairpin and the redox loop with the catalytic site.”
The findings represent a substantial advancement in our understanding of the photosynthesis process and have important potential applications in agriculture and biofuels.