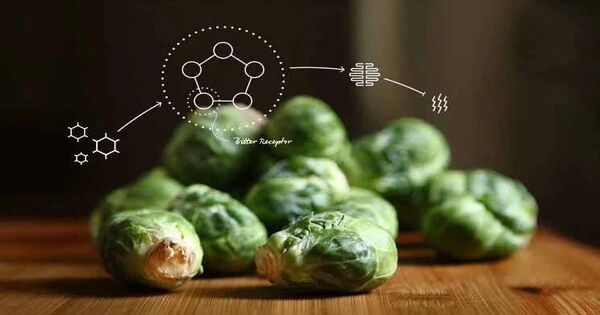
A new study has revealed the precise protein structure of the TAS2R14, a bitter taste receptor that allows us to sense bitter tastes. In addition to determining the structure of this taste receptor, the researchers discovered where bitter-tasting chemicals attach to TAS2R14 and how they activate it. These results could lead to the creation of medications that target taste receptors.
Humans can detect five distinct flavors using specialized sensors on our tongues called taste receptors: sour, sweet, umami, bitter, and salty. Aside from helping us to enjoy delectable foods, the sense of taste allows us to assess the chemical composition of food and protects us from swallowing hazardous chemicals.
Researchers at the UNC School of Medicine, including Bryan Roth, MD, PhD, the Michael Hooker Distinguished Professor of Pharmacology, and Yoojoong Kim, PhD, a postdoctoral researcher in the Roth Lab, recently set out to address one very basic question: “How exactly do we perceive bitter taste?”
Scientists know very little about the structural make up of sweet, bitter, and umami taste receptors. Using a combination of biochemical and computational methods, we now know the structure of the bitter taste receptor TAS2R14 and the mechanisms that initializes the sensation of bitter taste in our tongues.
Yoojoong Kim
A recent study published in Nature details the protein structure of the TAS2R14 bitter taste receptor. In addition to determining the structure of this taste receptor, the researchers discovered where bitter-tasting compounds bind to TAS2R14 and how they activate them, allowing us to experience bitter things.
“Scientists know very little about the structural make up of sweet, bitter, and umami taste receptors,” Kim stated. “Using a combination of biochemical and computational methods, we now know the structure of the bitter taste receptor TAS2R14 and the mechanisms that initializes the sensation of bitter taste in our tongues.”
This detailed information is important for discovering and designing drug candidates that can directly regulate taste receptors, with the potential to treat metabolic diseases such as obesity and diabetes.
From Chemicals to Electricity to Sensation
TAS2R14s are members of the G protein-coupled receptor (GPCR) family of bitter taste receptors. The receptors are attached to a protein known as a G protein. TAS2R14 stands out from the others in its family because it can identify more than 100 distinct substances known as bitter tastants.
Researchers discovered that bitter tastants link themselves to a particular location on TAS2R14 receptors known as an allosteric site. This causes the protein to reshape and activates the G protein that is associated to the receptor.
This sets off a chain of biochemical events within the taste receptor cell, activating the receptor. Activated receptors can then transmit signals to minuscule nerve fibers, which in turn travel through the cranial nerves in the face to the gustatory cortex, a region of the brain. Here, the signals are processed by the brain, which interprets them as bitterness. Naturally, this intricate signaling system happens almost instantly.
Cholesterol’s Role in Bitter Taste Reception
While working to define its structure, researchers found another unique feature of TAS2R14 — that cholesterol is giving it a helping hand in its activation.
“Cholesterol was residing in another binding site called the orthosteric pocket in TAS2R14, while the bitter tastant binds to the allosteric site,” said Kim. “Through molecular dynamics simulations, we also found that the cholesterol puts the receptor in a semi-active state, so it can be easily activated by the bitter tastant.”
Bile acids, which are produced in the liver, have similar chemical structures to cholesterol. Previous research has indicated that bile acids can bind and activate TAS2R14, but little is known about how and where they bind in the receptor.
Using their novel structure, researchers discovered that bile acids may bind to the same orthosteric pocket as cholesterol. While the actual role of bile acid or cholesterol in TAS2R14 is unknown, it may be involved in their metabolism or related to metabolic diseases such as obesity or diabetes.
How This Can Help Drug Development
The discovery of this novel allosteric binding site for bitter tasting substances is unique.
The allosteric binding region is located between TAS2R14 and its coupled G protein is called G-protein alpha. This region is critical to form a signaling complex, which helps to transfer the signal from the taste receptor to the G-protein to the taste receptor cells.
“In the future, this structure will be key to discovering and designing drug candidates that can directly regulate G proteins through the allosteric sites,” Kim stated. “We also have the ability to affect specific G-protein subtypes, like G-protein alpha or G-protein beta, rather than other G-protein pathways that we don’t want to cause any other side effects.”
Roth and Kim have made several new discoveries, but some raise more questions than answers. During a genomics investigation, they discovered that the TAS2R14 protein in association with the GI is expressed outside of the tongue, particularly in the cerebellum in the brain, the thyroid, and the pancreas. Future investigations are planned to elucidate the function of these proteins outside of the mouth.