A revolutionary study headed by researchers at Indiana University School of Medicine has given new insight into the genetic basis of Alzheimer’s disease. Based on human genetics research, the team discovered a significant mutation within a key gene acting in the brain’s immune cells, potentially increasing the risk of Alzheimer’s disease.
Gary Landreth, Ph.D., the Martin Professor of Alzheimer’s Research; Bruce Lamb, Ph.D., executive director of Stark Neuroscience Research Institute; Stephanie Bissel, Ph.D., assistant professor of genetics; Kwangsik Nho, Ph.D., associate professor of radiology and imaging sciences; and Adrian Oblak, Ph.D., assistant professor of radiology and imaging sciences were all part of the research team. Their findings were published in the journal Immunity recently.
Andy Tsai, PhD, a Medical Neurosciences Graduate Program graduate, was the main force behind the research, which included his PhD thesis. Tsai, who is now a postdoctoral fellow at Stanford University Medical School, has made substantial contributions to the understanding of Alzheimer’s disease.
The microglial response affects neurons which then affects the capacity to learn and form new memories. This represents a collaboration that could’ve only been achieved at Stark. We used human genetics to investigate and identify a mechanism, and indeed we have.
Gary Landreth
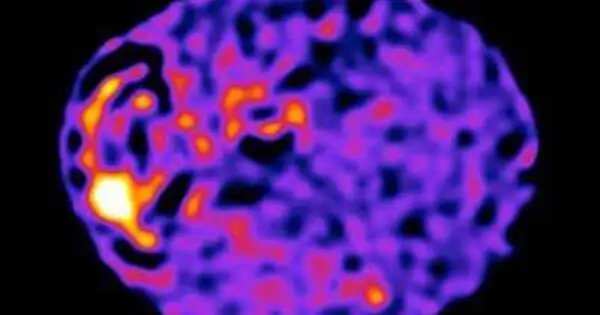
The analysis focused on the phospholipase C gamma 2 (PLCG2) gene, which is densely interwoven inside microglia and is essential for the brain’s immunological response. This genetic abnormality, uncovered by analyzing the gene’s biological functions, demonstrated the importance of unique rare variations. The M28L variation increased susceptibility to Alzheimer’s disease, whereas the P522R variant decreased risk.
The NIH-funded MODEL-AD Center’s innovative mouse models of Alzheimer’s disease allowed researchers to validate their findings. Immune cells with risk-lowering gene variants showed a decrease in amyloid plaque accumulation, whereas those with risk-raising variants showed an increase in plaque accumulation. The research identified distinct gene clusters that orchestrate changes in immune cell activity within microglia.
Microglia, which is frequently viewed as the brain’s first line of defense against infections, poisons, and damage, has received attention for its important role in disease vulnerability.
“The microglial response affects neurons which then affects the capacity to learn and form new memories,” Landreth stated.
Extensive collaboration inside the Stark Neurosciences Research Institute allowed for a thorough assessment of the gene’s implications. This includes comparing preclinical data from animal models to real-world human Alzheimer’s disease data.
“This represents a collaboration that could’ve only been achieved at Stark,” Landreth stated. “We used human genetics to investigate and identify a mechanism, and indeed we have.”
The study’s primary significance is in understanding the crucial function of microglial immune responses and their ability to influence disease risk, either favorably or adversely. This discovery has the potential to change our understanding of Alzheimer’s disease and pave the way for targeted therapies, which is a goal of the NIH-funded TREAT-AD Center.