An engineer is changing our understanding of what happens when water freezes by pushing the boundaries and getting the best look yet at tiny drops of water as they freeze. Though it is one of science’s greatest mysteries, the transformation of water into ice frequently escapes people’s minds because it is simply assumed to occur.
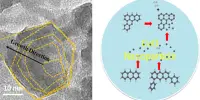
However, ice scientists such as Hadi Ghasemi, Cullen Associate Professor of Mechanical Engineering at the University of Houston, are closely studying how and why this occurs. Ghasemi is reporting the best look yet at the process of crystallization of water into ice at the molecular level: water-ice phase transformation down to 2 nm (nanometers) in diameter.
Then when Ghasemi examined these tiny particles, he made another discovery. He could break the limit of when water freezes and maintain the tiny droplets as a liquid by putting them in contact with soft interfaces, like gels or lipids.
Experimental probing of freezing temperature of few nanometer water droplets has been an unresolved challenge. Here, through newly developed metrologies, we have been able to probe freezing of water droplets from micron scale down to 2 nm scale.
Hadi Ghasemi
“We found that if a water droplet is in contact with a soft interface, freezing temperature could be significantly lower than on hard surfaces. Also, a few-nanometer water droplet could avoid freezing down to -44 C if it is in contact with a soft interface,” Ghasemi reports in Nature.
The limit of freezing temperature of a water droplet is -38 C. That is, any water droplet will freeze at some temperature between 0 C to -38 C. Below this temperature, freezing has been inevitable, until now.

The process of freezing such a tiny water droplet plays a critical role in the survival of animals in cold environments as a frozen water droplet inside a cell leads to the rupture of the cell and death. The process also plays a key role in climate prediction, cloud conditions, cryopreservation of organs, and technologies exposed to icing conditions such as aircraft and wind turbines.
“Experimental probing of freezing temperature of few nanometer water droplets has been an unresolved challenge. Here, through newly developed metrologies, we have been able to probe freezing of water droplets from micron scale down to 2 nm scale,” said Ghasemi.
Previously Ghasemi created an ice-repelling material for aerospace applications using a new concept called stress localization. His current findings contribute to a greater understanding of natural phenomena and provide guidelines for further design of anti-icing systems for aviation, wind energy and infrastructures and even cryopreservation systems.