INTRODUCTION:
Bangladesh (Mangifera indica L.) belongs to the family Anacardiaceae, is an important and popular fruit of Bangladesh. It has a unique position in respect of nutritional quality, taste, consumer’s preference etc., among the fifty kinds of fruits grown in Bangladesh (Ahmad, 1985). The fruit is believed to have originated in the Eastern India, Asam, Burma or in the Malayan region (Mukherjee, 1997).
Mango is now recognized as one of the choicest fruits in the world market for its excellent flavour, attractive color and delicious taste. It has medium calorific and high nutritional values. Carbohydrate content in ripe mango pulp is 16.9% (Salunkhe and Desai, 1984). Besides, mango contains appreciable quantity of provitamin A, vitamin C and soluble sugar (Samad et al., 1975). The unripe fruits contain nearly 50% more vitamin C than the ripe ones and in mineral content, mango holds an average position among fruits and in containing iron, unripe mango is the first and ripe fruit, about the 16th position among all major fruits (Hossain, 1989). The fruit has really of immense value in respect of money and prosperity. In Bangladesh it is called as “King of the fruit”.
Due to certain limitations of soil and climate conditions, the mango grows better in some selective areas of Bangladesh. The leading mango growing districts are Nawabgonj, Rajshahi, Rangpur, Dinajpur and Kushtia. It is grown over wide geographical areas particularly in India, Pakistan, Brazil, Mexico, the Philippines, Malayasia, Indonesia, Thailand, Burma and Srilanka. It has gained popularity in Egypt, South – East Africa, Hawaii and Northwest Australia. Producing 9.64 million tons of fruits from an area of 1.17 million hectare, India is the single largest producer of mangoes with approximately 66% of the world mango production (Jacobi et al., 2001). Mango ranks third among the tropical fruits grown in the world with a total production of million metric-tons (FAO, 2004). In Bangladesh, mango ranks first in terms of area and third in respect of production. According to BBS (2004), Bangladesh produces 190 thousand metric-tons of mangoes per annum from 50.61 thousand hectares of land. The average yield of mango in Bangladesh is only 3.72 t/ha (BBS, 2004). This yield is much lower compared to that of our neighboring countries like India (8.95 t/ha) (Gosh, 1998) and the Philippines (9.41 t/ha) (Espino and Javier, 1989).
Mango has been cultivated in this sub-continent from 4000 years ago (Candole, 1984). The wild mangoes particularly, M. sylvatica Roxb., is still found in the Chittagong Hill Tracts of Bangladesh. The mango varieties on the contrary belong to only M. indica L., which are predominantly monoembryonic in nature. In Bangladesh, only a small percentage of mango trees are grafted plants. The grafted mango plants are concentrated in a few places in the North-Western region of Bangladesh and mangoes of unknown varieties (seedling mangoes) are grown in the southern and other parts of Bangladesh (Bhuiyan, 1995). In Bangladesh 90% of existing mango plants are raised from seeds (Hossain, 1994) and for the lack of suitable variety Bangladesh now is in a decreasing trend in production (Sardar et al., 1995).
With the rapid increase in population, the nutritional as well as economic problems are getting worse parallely. To overcome these problems, development of mango variety(ies) by evaluation at different agro-climatic regions may be important. Replacement of all the inferiors by the superior varieties must be ensured. This requires a wide survey and collection of superior mango germplasm from home and abroad and thereafter their detailed evaluation under Bangladesh conditions or even for specific region is necessary.
The commercial mango varieties namely, Gopalbhog, Khirsapat, Langra, Fazli, Ashwina etc. have been selected from chance seedlings found in different parts of the Indian sub-continent. Still, there may be other superior chance seedling(s) available in the countryside of Bangladesh that remains unnoticed to the scientists. Some of these may be high yielder with superior quality and possess a regular bearing habit.
Recently, some exotic mango varieties including hybrid mangoes are gaining popularity in Bangladesh, which also could help to enrich our varietal lot. The eastern areas of Bangladesh do not produce commercially any reputed variety. Moreover, studies relating to the performance of such varieties grown in those areas are scanty. So, it is necessary to find out the qualitative performance of elite varieties in the eastern areas.
Due to lack of scientific information on physical and chemical characters of the fruits of different varieties of mango grown under Bangladesh conditions, a large quantity of this valuable fruits waste during the peak season, particularly in “on” years. So, these characters are very important to the consumers for selection of a particular variety.
Characterization is an important aspect for documentation of performance of the studied cultivars, which subsequently will help to introduce, select and improve the existing mango varieties. But, information regarding the morphological and phsio-morphological characteristics of mango varieties growing under different regions of Bangladesh is scanty. Only a few characters of a limited number of cultivars have been studied (Mollah and Siddique, 1973; Hossain and Talukdar 1974; Samad and Faruque, 1976; Bhuyan and Islam, 1989; Sardar et al., 1998). But variations in fruit characters as well as physio-morphological composition among the mango varieties may occur because of difference in soil and climate or because of variable rootstock used in propagation (Jagirder and Maniyar, 1960) or due to the propagation of varieties for improvement in mango (Naik et al., 1958). The study aims at giving information on the varietal characteristics. So, it becomes useful for the growers also in determining the cultivars to be grown.
In Bangladesh there are many genotypes of mango having diverse characters. These genotypes are available in the market under different local names without any uniformity and standardization in nomenclature. Moreover, no information on horticultural traits are available that can be used as basis for delineating and standardizing different available cultivars. The basic key to bring about the genetic upgrading of a crop is to utilize the available or created genetic variability.
The qualitative and quantitative improvement of plants depends on the available gene pool and its manipulation. Not only morphology based genetic diversity but also molecular markers studies enable the evaluation of genetic variation prior to the initiating of a new breeding programme. Molecular markers have appeared to be very useful tools for a better characterization of biodiversity. It has been used to reveal difference and relationship between taxa (Dore, 2001).
Isozymes (the gene product or enzymes of different gene loci) are the effective molecular marker. Battistini et al. (1991) stated that isozyme analysis by electrophoresis is a useful complementary tool in phenotypes. Isozyme analysis offers a possible alternative method for cultivar identification and had been successfully applied in several tree species (Durham et al., 1987).
With this in view, the present study was, therefore, carried out with the following objectives:
- to study the physio-morphological characteristics of 20 popular local, exotic and hybrid mango germplasm under Mymensingh conditions;
- to assess the performance of 20 mango germplasm for yield and yield contributing characters;
- to characterize the germplasms through isozyme technique; and
- to study the genetic diversity present in the germplasm collected form different parts of the country for future breeding program.
REVIEW OF LITERATURE:
Mango is a highly valued crop in Bangladesh, but information on the physio-morphological characteristics of different varieties under Mymensingh condition are scant. Several research works have been done in the world pertaining to the flower and fruit characteristics of mango. Some available research findings in this connection have been reviewed and presented here under the following heads.
Morphological characteristics of mango
Floral Biology:
Floral biology relates to the time of flower bud differentiation, time of flowering, blooming pattern, time of anthesis and dehiscence, period of availability of pollen grain and receptivity of stigma. Ali and Mazher (1960) described different time of emergence of flowers in various mango growing tracts viz. February – March in Multan, Pakistan; February in North India and late November and December in Southern India.
Valmayor (1962) consented that blooming period is dependent on the combination of environmental factors and condition of the plant. In most of the cases flower bud differentiation occurs between October to December under the climatic condition of India as reported by Singh (1968). The flowering time in mango is dependent on the climatic factors prevailing in an area; low temperature may extend it, whereas higher temperature may shorten it (Majumder and Sharma, 1990).
Sardar and Hossain (1993) stated that under the climatic condition of Rajshahi the germplasm Amrapali, Mallika and Rad flowered in the 3rd – 4th week of February.
Sardar et al. (1998) studied the physico-morphological characteristics of ten mango cultivars and noted that under the climatic condition of Rajshahi, Kohitoor and Fazli were the earliest (15.1.93) and the latest (31.1.93), respectively to flower initiate. Moreover, flowering duration of the different cultivars ranged from 25 to 35 days.
Hossain and Talukdar (1974) studied the panicle characteristics where its color varied from deep to light green. They also noticed distinctive color in certain varieties such as pink to light pink and chocolate.
Singh (1954) reported that the number of hermaphrodite flowers is the least in the upper part of the panicle but the percentage is the highest.
Hossain et al. (1977) studied flush characters and floral morphology of mango which revealed that the panicle length ranged from 13.97 to 27.68 cm. they also observed the highest number of total (1903) and male flowers (93.23%) from Gopalbhog. Among the studied varieties, Khirsapat produced the highest percentage of bisexual (18.88) and the lowest percentage of male flowers (81.12).
Singh (1978) reported that under North Indian conditions percentages of perfect flowers in the variety Dashehari and Langra were 30.6 and 69.8, respectively. On the other hand, in South Indian mangoes it varied from 16.41% Neelum to 3.17% in Allampur Beneshan.
An investigation was carried out by Bhuyan and Islam (1989) to study the physico-morphological characteristics of five popular mango cultivars. They reported that Fazli and Ashwina were the earliest (27.1.86) and the latest (4.2.86), respectively in respect of flower initiation. Flowering duration of the cultivars ranged from 18 (Khirsapat) to 25 days (Ashwina). Gopalbhog had the maximum number of panicles (2.13) per shoot, whereas the maximum number of main branches (26.13) per panicle was recorded in Fazli, Khirsapat and Ashwina had the longest panicles (31.67 cm).
Haque et al. (1993) evaluated 20 elite mango cultivars at southern Bangladesh and stated the cvs. Baromashi flowered earlier (2nd half of December) than the others. The number of panicles per shoot was higher in the cvs. Badshabhog and Chondonkhos (5.0) and lower in Lata and Sofeda (1.0). The lengths of panicles were varied from 26.6 to 46.0 cm. The cv. Chondonkhos had higher number of primary branches (63) per panicle. They also observed greater male and bisexual flower ration in Brindabani (19.1) and Safeda (11.1).
An investigation was done by Islam et al. (1995) at Mango Research Station, Nawabgonj on floral characteristics of eight mango cultivars and stated that flowers bud emergence of all the cultivars took place in January. The duration of flower bud emergence ranged from 22.5 to 29.09 days. They further noted the highest number of panicles (2.13) per shoot in Gourjeet and lower (1.0) in Fonia. The length of panicle varied from 27.79 to 33.77 cm. they also estimated the highest number of main branches (29.56) per panicle from Satiarkara and the lowest in Fonia (14.50).
A study was conducted by Iqbal et al. (1995) on the performance of exotic mango germplasm and recorded the highest male flower (93.39%) from the germplasm Carabao and the lowest from Kent (69.86%). Bisexual flowers of the 18 different germplasm were in the range of 6.61 to 30.46%. Mango flowers are borne on terminal of pyramidal panicles, glabrous or pubescent; the inflorescence is widely branched, usually densely flowered with hundreds of small flowers. Both male and perfect flowers are found within a single inflorescence. The ration of male to perfect flowers is strongly influenced by environmental and cultural factors (Mukherjee, 1997).
Fruit set and fruit drop:
Fruit set implies that the flower has been pollinated and fertilized and in consequence, the ovary and accessory tissues will be capable of growing into a fruit. Singh (1978) reported that fruit set and ultimate retention per panicle are much higher in medium and late emerged panicles as compared with the early ones and it is a varietal character depending upon time of flowering, efficient cross-pollination and fruit drop intensity. Initial fruit set in mango is directly related to the proportion of perfect flowers, although the final fruit set dose not necessarily depend on the ration (Iyer et al., 1989).
An investigation was carried out by Uddin et al. (1995) to study the performance of six primarily selected lines of mango. They recorded the highest fruit set per panicle (19.33) conducted an experiment to evaluate the performance of 18 exotic mango germplasm. They stated that number of fruit set per panicle varied from 1.22 to 37.53. Moreover, they recorded the highest (2.20) fruit-harvest per panicle from Amrapali and the lowest (1.00) from Kent.
Singh (1954) stated that the development of fruit in varieties ‘Langra’ and ‘Dashehari’ stated in the last week of March and is completed by the end of 2nd week of June. On the other hand, fruit drop in various stages is a common problem in mango fruit. It may occur due to lack of pollination, abnormalities of floral parts, formation of abscission layer, lack of nutrient and water in soil.
Jawanda and Singh (1961) gained experience on the fruit set and extent of fruit drop and reported that on an average only 0.4 per cent fruit per panicle was obtained during the harvesting time. They also reported that maximum fruit drop took place in April.
Chandha and Singh (1965) found increased fruit dropping during the rapid development of ovary at the initial stage. According to them, the varieties Langra and Dashehari ceased dropping when the fruit attained 90.95 per cent of total length.
The natural fruit drop in mango is rather too high, amounting to about 99 per cent at various stages of growth, more especially during the initial four weeks (Singh, 1968).
Hossain and Talukdar (1974) studied the characteristics of Bangladeshi mangoes grown at Rajshahi and recorded the maximum number of fruit set per panicle in Ranipasand (41.80) and the lowest in Kishanbhog (5.20). They also observed the increased fruit dropping (up to 98.47%) at pea stage. At marble stage they recorded maximum fruit dropping in Surmai Fazli (47.81%) and minimum is Dilsad (1.93%). Fruit dropping was minimum in mature stage, which was in the range between 0.10 and 3.26%. Finally they narrated that fruit harvest per panicle varied from 0.17 to 7.54%.
An experiment was conducted by Islam et al. (1995) on fruit characteristics and observed that the percentage of fruit dropping over initial fruit set ranged from 58.61 (Rajbhog) to 97.77 (Motichur).
Harvesting time:
In India, Pandey (1984) stated that the harvesting time of Mallika and Amrapali was 3rd week of July. On the other hand Sardar and Hossain (1993) reported that under the climatic conditions of Rajshahi the germplasm Amrapali, Mallika and Rad were harvested in the second week of July. Hossain (1998) reported that mango under Bangladesh conditions takes about four to six month to reach maturity after flowering.
Bhuyan and Islam (1989) carried out an experiment at Mango research Station, Nawabgonj and reported that the fruits of all the studied cultivars were harvested between 31.5.86 to 21.7.86. Gopalbhog was the earliest and Ashwina, the latest in harvesting time. They also noted that Ashwina took maximum time (167 days) for maturity from flower initiation.
According to Haque et al. (1993) harvesting time varied from 118 – 163 days at southern region of Bangladesh where they evaluated 20 cultivars of mango. Gopalbhog was the earliest to harvest (118 days) and Baromashi was the latest (163 days).
An experiment was conducted by Isalm et al. (1995) on physio-morphological characteristics of eight mango cultivars and noted that Satiarkara and Kuapahari were early and late maturing cultivars, respectively. The days required for fruit maturity of different cultivars range from 86-117.
In Panjab, India, Sharma and Josan (1995) reported that fruits of Langra matured during the second week of July whereas those of Mallika and Amrapali in third week of July.
Sardar et al. (1998) narrated that Gopalbhog and Ashwina were early and late cultivar, respectively in respect of harvesting time. Ashwina took the maximum number of days (134) for maturity from flowering and the shortest period (92 days) was taken by Gopalbhog.
Yield:
The yield of mango varied from area to area, season and variety to variety. Majumder and Sharma (1990) reported that the yield is a highly variable factor depending upon the cultivars and age of the plants, climatic conditions, incidence of the pests and diseases etc.
Singh (1978) reported that at the start of bearing the yield may be low as 10 to 15 fruits (2 to 3 kg) per tree and rising to 50 to 75 frits (10 to 15 kg) in subsequent years.
Sardar et al. (1995) studied the performance of introduced mango germplasm under Bangladesh conditions and recorded highest yield 150 fruits (17.0 kg/plant) from the variety Amrapali. They further recorded the lowest yield 30 fruits (3.5 kg/plant) from Pahutan.
Uddin et al. (1995) investigated the performance of six primarily selected mango lines and reported that mango line NMS-035 produced highest yield (44.1 kg/plant). On the contrary, NMS-027 produced the minimum lowest yield (3.8 kg/plant) among the lines studied.
Another experiment on the performance of introduced mango germplasm under Bangladesh conditions was carried out by Bhuyan and Guha (1995). The highest yield per tree (20.36 kg) was obtained from the germplasm Ruby and the lowest (0.89 kg) from M-3896.
Physical characteristics of fruits:
Mullah and Siddique (1973) studied the physiological characteristics of some mango varieties of Bangladesh and found that the variety Gobindabhog was the highest (620.42 g) in size while Langra had the highest pulp content (77.47%). Qunatitative characters like length, breadth and thickness of fruits varied from 7.21 to 14.00, 6.15 to 9.70 and 5.96 to 8.66 cm, respectively.
In Bangalore, India, Lodh et al. (1974) evaluated the physiological characteristics of mango fruits and pointed out that the weight varied from 209 to 622 g. the length and thickness of fruits ranged between 10.06 to 13.59 and 5.96 to 9.31 cm, respectively. A wide variation was also observed in respect of pulp (66.0 to 75.0), peel (13.0 to 20.0) and stone (12.0 to 16.0) percentage.
Palaniswamy et al. (1974) noted that mango fruit weight ranged between 101.0 to 670.0 g and pulp percentage 53 to 83 among the 29 cultivars of Tamil Nadu, India.
Samad (1975) examined the physical and bio-chemical characteristics of 10 mango fruits and pointed out that the fruit of Fazli was the largest (512.83 g) in size and contained the highest percentage of pulp (388.57 g).
While investigating the South Indian varieties in Northern India, Prasad (1977) recorded the maximum fruit size (11.75 × 7.62 cm) and weight (318.32 g) in the variety Banglora. A quite large fruit was also recorded in Allampur Baneshan (10.97 × 7.50 cm), Janardanpasand (11.86 × 7.32 cm) as well as Peddarasam (12.13 × 6.12 cm).
Fruit characteristics, such as size shape and pulp of different mango varieties under the climatic condition of Rajshahi was investigated by Hossain and Talukdar (1974). Data indicated that the variety Fazli had the heaviest fruit (683.27 g) and the lightest was from the Bira (113.86 g). The pulp weight ranged from only 66.63 to as high as 538.69 g. Stone characteristics in respect of length, breadth and weight were also studied. The stone length varied from 3.81 to 12.32 cm. A wide variation was observed in stone breadth among the studied varieties as it ranged from 2.94 to 7.41 cm. Stone weight was the highest (144.58 g) in Dilsad but the lowest (13.99 g) in Goplabhog. Maximum pulp to stone ratio was found in Hazi Langra (1:0.44) whereas it was the lowest (1:0.05) in Fonia.
Pandey (1984) reported that ripe fruits of Carabao, Irwin, Kent, Keitt, Mallika, Pahutan, Palmer and Amrapali were yellow, orange yellow, greenish yellow, bright yellow, apricot yellow, light yellow, light greenish to orange yellow and apricot yellow color, respectively.
In a study on physiological characteristics of some mango varieties, Bhuyan and Islam (1986) recorded the highest fruit weight (1014.45 g) in Fazli and the lowest (202.88 g) in Kude-Khirsapat. Wide range of variability among the varieties was recorded in fruit size, percentage of edible (64.94-81.49) and non-edible portions (18.51-35.06), stone size and thickness of fruits (6.02-8.92 cm). Fazli had the longest fruit (17.70 cm) and the shortest (8.16 cm) in Satiarkara. The highest breadth of the fruit (10.74 cm) was also observed in Fazli and that of the lowest (6.54 cm) in Fonia. The stone percentage of Gopalbhog was the highest (19.25) and that of Fazli was the lowest (8.07). The peel percentage varied from 8.87 to 17.32 among the studied varieties. The stone length ranged between 6.50 to 14.80 cm and breadth from 3.38 to 6.50 cm.
In Bihar, India, Syamal and Mishra (1987) reported that Fazli produced the heaviest fruits (506 g) followed by Langra (310 g) and Paharpur Sinduri (294 g). Fazli and Langra also had high pulp contents.
Ghose and Hossain (1988) studied the physiological characteristics of 10 mango varieties at Joydevpur and narrated that mango varieties under study varied greatly in shape, size, skin color and weight. The variety Kalibhog produced the largest fruit (13.1 × 8.6 × 8.6 cm) while Brindaboni the smallest one (7.1 × 5.4 × 4.9 cm3). Again, Kalibhog had the maximum fruit weight (655.0 g) but Brindaboni the minimum one (106.0 g). Further more; Kalibhog contained the highest pulp (78.5%), the lowest stone (9.8%) as well as peel (11.7%). Kalibhog was considered superior one in respect of size, shape, edible portion, taste and finally the flavor to all the concerned 10 varieties.
An investigation was carried out by Saha and Hossain (1988) to evaluate the fruit characteristics of 11 mango cultivars. They reported that skin color at ripe stage varied greatly from yellowish green to bright yellow. Wide variation was also recorded in shape of fruits. Pulp color ranged fro yellow to red. They also stated that quantitative characters namely length, breadth and thickness of fruits varied from 7.6 to 14.1, 5.9 to 7.3 and 5.5 to 8.2 cm, respectively. The length, breadth and thickness of stones varied from 6.0 to 11.9, 3.1 to 5.0 and 1.7 to 2.5 cm, respectively. A significant difference was found in respect of weight of fruit (137.2 to 608.3 g) percentage of pulp (50.1 to 81.5), stone (9.3 to 23.4) and peel (9.2 to 27.0). Fruits of Fazli were better in respect of size and percent edible portion. On the other hand, Gopalbhog was better in respect of pulp color and taste.
In an experiment in RARS, Hathazari, Chittagong, Ahmad et al. (1989) evaluated ten relatively small-fruited mango varieties and narrated that the mean weight of fruits was 151.2 g, with proportion of pulp, skin, and stone being 68.4, 15.9 and 15.8 percent, respectively. The heaviest fruits were Kalia (214.8 g), Deori (175.5 g) and Sultan Pasand (176.6 g). The highest proportion of pulp was in Narikeli (75.9%), Lata (74.8%), Bombai (71.8%) and Sultan Pasand (71.6%). The proportion of skin of Bombai Dr. King was the highest (20.5%) whereas Narkeli was the lowest (11.7%).
Bhyan and Islam (1989) studied the physiological characteristics of some popular mango cultivars and reported that fruit weight varied from 208.0 to 654.44 g. the greatest fruit weight (654.44 g) was observed in Fazli, which had the greatest pulp weight too. The maximum percentage (77.82) of edible portion was recorded in Ashwina and the minimum (59.13%) in Gopalbhog.
Sardar et al. (1995) studied the performance of five introduced mango varieties namely Amrapali, Mallika, Carabao, Pahutan and Rad. They stated that under Bangladesh conditions the fruits of all these varieties were excellent in appearance. Mallika produced the biggest fruit (463.4 g) followed by Rad (230.5 g). The maximum edible portion was observed in Mallika (76.1%) followed by Rad (73.4%) and Pahutan (73.01%).
Islam et al. (1990) in an experiment with 15 mango varieties at mango research station Nawabgonj, found wide range of variation among the varieties for fruit shape (long and oblong), fruit weight (165 to 460 g), skin color, pulp color, stone weight (20 to 65 g), TSS (14.5 to 25.3%) and taste.
Islam et al. (1992) conducted an experiment on physiological characteristics of ten mango cultivars and narrated that the cultivar Dudshar produced the largest fruit size (13.4 × 7.0 × 6.2 cm) and Kude-Khirsapat, the smallest (8.3 × 6.6 × 6.0 cm3). Krishnachura had maximum fruit weight (425 g) and Khudekhirsapat the minimum (212 g). Percent edible portion was the highest in Khudekhirsapat (73) and the lowest in Satiarkara (65.2). Fruits of Fonia, Gourjeet, Satiarkara and Kudekhirsapat had thin skin while fruits of Krishnachura were thick skinned. Pulp color of Gourjeet and Rajbhog was deep yellow and that of Kanchamitha was light yellow. Pulp color of fruits of the remaining cultivars was yellow.
An evaluation was done by Haque et al. (1993) at southern Bangladesh to find out the varietal characteristics and color of ripe fruits. Maximum fruits turned to yellow or greenish yellow during ripening while the cvs. Kohitur and Summerbehest turned to red and reddish yellow. Length of the fruit ranged from 10 to 17 cm and the highest (14.7) breadth was noted in the cv. Badshabhog whereas the shortest (8.5 cm) in cv. Baromashi. Fruit thickness varied from 4.8 to 8.6 cm. Bigger and heavier fruits were found in Mohonbhog (670 g) and Fazli (615 g). Fruit weight ranged from 159 to 670 g. Significant differences were observed in case of stone length (5.0 to 11.5 cm), breadth (3.0 to 6.0 cm) and thickness (1.7 to 3.0 cm). Stone weight varied from 14.0 to 70.0 g among the cultivars.
Saha et al. (1994) in an experiment with some superior late mango varieties, found wide range of variation among the varieties for fruit characters like fruit weight (149 to 250 g), fruit length (7.9 to 20.5 cm), fruit breadth (7.1 to 12.4 cm), fruit shape, fruit color, pulp color and taste.
An experiment was conducted by Iqbal et al. (1995) to investigate the performance of exotic mango germplasm. Marked variation in fruit characteristics was observed. Keitt produced the biggest fruit (675.8 g) while the smallest (62 g) was found in M-3809. Rad had the highest edible portion (79.06%) whereas Agmamashu had the lowest (60.47%). Again Kent produced heaviest stone (47.08 g) but Rad produced the longest stone (1056 cm). Stone characteristics like length, breadth and thickness were varied from 5.35 to 10.56, 2.23 to 4.28 and 1.17 to 2.17 cm, respectively.
Saha et al. (1995) evaluated 157 accessions of 6 mango varieties viz. Gopalbhog, Himsagar, Khirsapat, Bombay, Langra and Surjapuri and found variation among and between varieties for different characters. Fruit weight ranged from 172 to 600 g, and the largest fruits of selected varieties were in the range of 418 to 600 g, stone weight ranged from 25 to 110 g and TSS ranged from 14.2 to 23.3%. Variation was also observed in qualitative characters like fruit shape (Ovate to oblong), fruit color (green to yellow), pulp color (light to radish) and taste (moderate to vary sweet).
In semi-arid region of Moharastra, India, Chaudhari et al. (1997) evaluated South Indian mango varieties and narrated that the quantitative characters like length and diameter of fruits varied from 6.7 to 15.0 and 5.5 to 10.2 cm, respectively. Less variation was observed in percentage of pulp (46.5 to 68.0), peel (14.3 to 28.0) and stone (16.1 to 27.8).
Fruit color at maturity is genotype dependent. Fruit of Bombay green is green Carobao, Manila, Mulgoa and Arumanis are greenish-yellow and Haden, Keitt and Tomy Atkins have a striking red blush as reported by Mukherjee (1997).
Sardar et al. (1998) observed wide range of variability in respect of different physiological characteristics of mango fruits. Skin and pulp color of ripe fruits varied from green to yellow and yellow to orange, respectively. The largest fruit (678.3 g) was recorded in Fazli and the smallest (126.9 g) Bhabani. Fazli had the longest fruit (15.5 cm) and the shortest (7.6 cm) in Ilsapeti. Fruit breadth and thickness varied from 5.5 to 8.9 and 5.0 to 8.2 cm, respectively. The longest (11.4 cm) and the widest (5.7 cm) stone were found in Fazli whereas that to Ilsapeti was shortest (5.6 cm) as well as narrowest (3.1 cm). Thickness of stone was the highest (3.2 cm) in Ashwina and the lowest in Ilsapeti (1.8 cm). Percentage of edible and non-edible portion varied from 58.5 to 75.1 and 24.9 to 41.5, respectively. Furthermore, Ilsapeti had the highest stone portion (26.2%) and the lowest in Kishanbhog (12.5%).
Hossain et al. (2001) conducted an experiment with three varieties of mango and find out the physio-morphological and chemical compositional differences in the fruits. Amrapali possessed highest pulp with skin (87.12%) and lowest stone (11.88%) but the fresh fruit weight was minimum (221.33 g). The variety of Bishawanath had the highest fresh weight (256.0 g) and stone (18.67%) and lowest in pulp with skin (81.33%), pulp stone ratio (4.4:1) and keeping quality (8.75 days). Keeping quality was maximum in Amrapali (12.5 days). Total sugar (26.85%), TSS (23.50%) and pH of pulp (6.0) were maximum in Amrapali, but Bishawanath showed maximum titratable acidity (0.87%) and Vitamin C (14.20 mg/100 g). So, Amrapali was superior in respect of all characters to other varieties.
Isozyme Study in Mango :
Genetic diversity for the improvement of the crop has been stressed in both cross and self-pollinated crops (Gaur et al., 1978). The quantification of genetic diversity through biometrical procedures has made it possible choose genetically diverse parents for a successful hybridization programme (Jain et al., 1975). Tomooka (1991) reported that evaluation of genetic diversity is important to know the source of gene for a particular trait within the available germplasm.
Electrophoretic separation of enzymes has been widely used, both in taxonomic and genetic studies of different crops (Shannon, 1968). In this respect, all the individuals of a given genotype must have the same allele(s) at all loci. This occurs in clonally propagated material and in homozygous and F1 hybrid plants (Messegur et al., 1987).
Isozymes are characterized by co-dominant expression, making them excellent markers for hybrid identification. The utility of isozyme analysis has been successfully used for demonstrating intra and inter-specific hybrid identification in mango and many other fruit trees (Torres, 1983; Moore and Litz, 1984; Parfitt et al., 1985; Jarret and Litz, 1986; Chaparro et al., 1989). McGranahan et al. (1986) demonstrated that isozymes were also excellent markers for identifying inter-generic hybrids of Juglans. Isozyme markers are advantageous over conventional morphological markers because they allow identification of these hybrids at the seedling stage, permitting early selection of desirable individuals.
Chen et al. (1988) presented details of construction and operation of a kit. The techniques and results of isoenzymic experiments on melon, cucumber, tomato, Capsieum, Carica, Citrus and mango using peroxidase and phosphoglucose isomerase [glucose-6-phosphate isomerase] are presented. These include the use of isoenzymes as genetic markers in melon and for species/variety identification in citrus.
Torres et al. (1989) illustrated the use of isoenzymes for the genetic study of tree fruits is illustrated for apples, pears, peaches, mulberries, figs, olives, citrus, avocados, date palms, mangoes, cherimoysa (Annona cherimola).
Schnell et al. (1992) examined five isozyme systems to detect zygotic seedlings from five polyembryonic cultivars of mango (Mangifera indica). Significant difference were found between cultivars (chi 2 = 35.5) for the percentage of zygotic and nucellar seedlings. The percentages were 4, 24 and 36 in Sabre, Turpentine and Madoe, respectively. Three of eight rootstock mother trees of Turpentine were determined to be off-types.
According to Yadev (1997), isozymes analyses of 200 polyembryonic mango varieties revealed that there were 34 allels which showed monomorphism while 19 showed polymorphism.
Eiadthong et al. (1998) analyzed leaf extracts by using starch gel electrophoresis, leaf extracts of 58 mango (Mangifera indica) for variation of isocitrate dehydrogenase (C 1.1.1.42), phosphoglucose isomerase [glucose-6-phosphate isomerase] (PGI, EC 5.3.1.9) and alcohol dehydrogenase (ADH EC 1.1.1.1 isozymes) polymorphism was found in all enzymes system examined and intracultivar variation of the same cultivar name collected at different locations were confirmed in banding patterns. No correlation between the polyembryonic and monoembryonic groups was found. Banding patterns of the enzyme systems allowed classification of Thai cultivars into 14 groups. Nam Dok Mai, Khieo Sawoei, Falan Nong Seang, Arumanis, Kalon Thong, Edward groups consisted of 13, 11, 10, 8, 5 and 2 cultivars, respectively. One cultivar was identified for Carabao, Davis Haden, Haden, Lippens, Pope, Talubnak and Zill groups.
Yamamato et al. (1998) described the methods to determine isozymes and leaf genotype and subsequent classification into species. Isozyme analysis consisted of glutamate oxaloacetic transaminase (GOT) and peroxidase (Px) which was related to sample genotype.
Decha et al. (2000) studied isozyme characteristics of polyacrylamide gel electrophoresis technique. Enzyme from 7 month-old leaves was extracted by tris-buffer 0.1 M., pH 8.2. Twenty-two percentage of gel concentration was suitable for acid phosphates and esterase, while 7.5 percent concentration was suitable for peroxidase. It was found that the three isozyme systems; acid phosphatase, esterase and peroxidase separately could identify 52 clones into 10, 4 and 15 groups respectively. Using the combination of 3 isozyme systems, the 52 clones could be grouped into 20 clones and other 9 groups.
Gill et al. (2002) studied the peroxidase and amylase enzymatic patterns and stated that isoenzyme studies can be used as a powerful tool for the characterization of in vitro raised nucellar seedlings of mango as well as for other fruit plants.
It is revealed from the review of literature that a wide variation or differences are present in respect of morphological traits and genetic divergence of mango.
MATERIALS AND METHODS:
This chapter deals with the materials used and methods followed in conducting the experiment. It includes a brief description of location of the experimental site, climate, design of the experiment, statistical analysis and materials used for the experiment. The details of the experiment are described below:
Location of the experimental site:
The present experiment was carried out at the Germplasm center of Fruit Tree Improvement Project (FTIP), Department of Horticulture and the Laboratory of Department of Genetics and Plant Breeding, BangladeshAgriculturalUniversity, Mymensingh with 20 germplasm of mango, collected from home and abroad during March 2004 to August 2005.
Climate:
The experiment area was under the sub-tropical climate, characterized by heavy rainfall, high humidity, high temperature, short clear sunshine during the month from April to September and scanty rainfall, low humidity low temperature, long clear sunshine and short day during the rest period of the year. Details of the weather data collected from weather yard, Department of Irrigation and Water Management, BAU, Mymensingh are presented in Appendix I.
Soil:
The soil of experiment area was silty-loam in texture belonging to the Old Brahmaputra Flood Plain (FAO, 1971). It was a medium high land, fertile, well drained and slightly acidic with pH varying from 5.5 to 6.8.
Materials:
A large number of mango varieties of varying ages have grown at the Germplasm (GP) center and these varieties were planted around 1991’s. Among them, about ten to twelve years old twenty indigenous, exotic and hybrid mango plants (Table 1) were selected for the study.
Table 1. Mango varieties selected for the present study
| Sl. No. | Genotype | Accession | Name of the varieties | Source/origin |
1 | G1 | MI – 001 | Rad | The Philippines |
2 | G2 | MI – 002 | Farooquebhog | Bangladesh |
3 | G3 | MI – 004 | Neelumbori | Bangladesh |
4 | G4 | MI – 008 | Chausa | India |
5 | G5 | MI – 009 | Mallika | India |
6 | G6 | MI – 016 | Tomy-atakin | USA (Florida) |
7 | G7 | MI – 019 | Hybrid -10 | India |
8 | G8 | MI – 020 | Shindhu | India |
9 | G9 | MI – 022 | Mixed special | India |
10 | G10 | MI – 023 | Gopalbhog | Bangladesh |
11 | G11 | MI – 024 | Surmai Fazli | Bangladesh |
12 | G12 | MI – 025 | Langra | Bangladesh |
13 | G13 | MI – 026 | Khirsapat | Bangladesh |
14 | G14 | MI – 028 | Amrapali | India |
15 | G15 | MI – 030 | Maldaha Fazli | Bangladesh |
16 | G16 | MI – 043 | Pahlum | Thailand |
17 | G17 | MI – 044 | Kent | USA (Florida) |
18 | G18 | MI – 045 | Keitt | USA (Florida) |
19 | G19 | MI – 047 | Kalibhog | Bangladesh |
20 | G20 | MI – 048 | Mohananda | Released by BARI |
These 20 germplasm of mango were considered as the experimental treatments. The total number of trees was 60. From each germplasm three trees were selected, which were considered as the three replications. Both the distances between plant to plant and row to row were 6 m. The experiment was divided onto three separate parts viz.
- Morphological characteristics of 20 mango germplasm
- Physical characteristics of 20 mango germplasm, and
- Characterization of 20 mango germplasm using peroxidase enzyme
A brief description of the germplasm are given below:
MI – 001 (Rad): It is an exotic as well as semi-dwarf variety. Fruits are long, medium in size, very sweet in taste, delightful flavor.
MI – 002 (Farooquebhog): It is a chance seedling selection, collected from Mymensingh district. Fruits are medium in size, moderately sweet and the bearing is moderate and fairly regular.
MI – 004 (Neelumbori): It is a local dwarf variety; fruits are obong to oval shaped, medium in size, sweet in taste. It is a late variety.
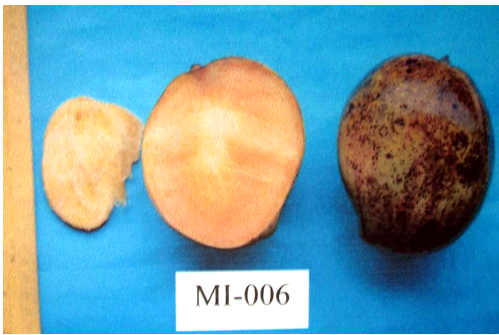
MI – 006 (Neelumboti): It is a local dwarf variety. Fruit are oblong oval in shape, medium sized, sweet in taste. It is also a late variety.
MI – 008 (Chausa): It is an exotic mid-season variety, fruits are oval shaped, medium in size and sweet in taste.
MI – 009 (Mallika): It is a hybrid variety. Developed from crossing between Neelum × Dashehari, fruits are oblong shaped, large in size and sweet in taste.
MI – 016 (Tommy Atkin): It is an exotic semi-dwarf variety. Fruits are large in size, moderately sweet, late variety.
MI – 019 (Hybrid – 10): It is a dwarf mid-season variety. It is also a hybrid of the cross between Baganpali × Alphonso. Fruits are large in size, roundish and regular bearing variety.
MI – 020 (Shindhu): It is also a dwarf mid-season variety. Fruits are small in size, roundish and regular bearing variety.
MI – 023 (Gopalbhog): It is an early variety. Fruits are medium in size; roundish in shape and sweet in taste and much juicy.
MI – 024 (Surmai Fazli): It is a popular and late variety. Fruits are large in size and sweet in taste and juicy.
MI – 025 (Langra): It is the most commercial variety of Bangladesh; fruits are ovalish oblong in shape, excellent quality in taste and more juicy. It is a mid-season variety.
MI – 026 (Khirsapat): It is an early and commercial variety. Fruits are round shaped, sweet in taste and juicy.
MI – 028 (Amrapali): It is a hybrid of the cross between Dashehari × Neelum; dwarf plants, late variety, fruits are medium in size, very sweet and juicy.
MI – 030 (Maldah Fazli): It is an early variety. Fruits are medium in size, roundish, sweet in taste and juicy.
MI – 043 (Pahlam): It is a late variety. Fruits are medium in size, oblong in shape, sweet in taste and juicy.
MI – 044 (Kent): It is a mid-season variety. Fruits are large insize, oval in shape, sweet in taste and juicy.
MI – 045 (Keitt): It is a mid-season variety. Large sized fruits. Oval in shape, sweet in taste and thick skin.
MI – 047 (Kalibhog): It is a mid season variety. Fruits are small, round in shape and sweet in taste.
MI – 048 (BARI Aam -1, Mohananda): It is also a late variety. Large in size, oblong in shape, sweet in taste and juicy.
Experimental design
The experiment was laid out in Randomized Complete Block Design (RCBD) with three replications.
Methods used for studying the morphological characters:
Leaf characteristics:
The characteristics of leaves studied were relating to length, breadth, petiole length. Measurements were made with the help of metre scale and the data were recorded in cm. Leaf shape, leaf margin and leaf tip were also recorded by using catalogue of mange germplasm, IIHR.
Leaf shape:
Ten leaves were selected randomly from the plant of each accession. The shape of leaf was recorded by visual observation. The shape of the leaves was classified into oblong-lanceolate, lanceolate and elliptic lanceolate.
Leaf tip:
The tip of the leaves was recorded by visual observation after selecting ten leaves from each accession. The leaf tips were classified into obtuse, acute and acuminate.
Leaf margin
The margin of leaves was recorded by visual observation after selecting ten leaves randomly from each accession. The leaf margins were classified into flat, wavy, folded and crinkled.
Leaf length:
The length of the leaves was measured by measuring scale and expressed in cm.
Leaf breadth:
The breadth of leaves were also measured by a measuring scale and expressed in cm.
Petiole length:
The length of petioles was measured by a measuring scale from the start of petiole to the start of lamina and expressed in cm.
Flower characteristics:
Time of flower bud emergence:
Date of emergence of flower bud was determined by daily observation on each of the selected trees during flowering season.
Time of first panicle emergence:
The date of panicle emergence was recorded by observing developed flower bud.
Time of first flower opening:
Date of first flower opening was determined by daily observation on each of the selected trees during flowering season.
Time of full bloom:
Total number of panicles per selected flowering shoot was counted.
Length and breadth of panicle:
Ten panicles other than the selected ones from each of the plants were collected randomly and kept into polythene bags and were taken to the laboratory to study the floral characteristics. The average length and breadth of 10 panicles were measured by a measuring scale.
Number of main branch per panicle:
Number of main branches per panicle was counted.
Shape of the panicle:
It was recorded according to IBPGR mango descriptor.
Position of the panicle:
It was determined by using a catalogue of mango germplasm, IIHR.
Panicle color:
Panicle color was determined by using a catalogue of mango germplasm, IIHR.
Leafy bracts:
It was determined by using a catalogue of mango germplasm, IIHR.
Flower diameter (mm):
It was measured by a measuring scale
Type of flower:
It was recorded by using a catalogue of mango germplasm, IIHR.
Nature of disc:
It was recorded by using a catalogue of mango germplasm, IIHR.
Number of stamen:
It was recorded according to IIHR mango catalogue germplasm.
Per cent male, bisexual and unopened flower:
Five panicles from each of trees were selected and the flowers were separated as male, bisexual and unopened. The total number of flowers was also recorded and percentage of different flowers was computed.
Fruits set per panicle:
Initial and final fruit set was recorded through daily inspection. The time of fruit set was the one when an ovary turns to green and attains the size of a normal pea.
Fruit harvest (%) per panicle:
Number of fruits per panicle was recorded and the percentage of harvested fruit was also computed.
Harvesting time:
Harvesting time was determined by the incidence of dropping of few normal ripe fruits.
Days to maturity:
Time required from flowering to harvest of fruits was also recorded.
Number of harvested fruits:
Total number of mature fruits per plant was recorded.
Weight of harvested fruits:
Immediately after harvesting the fruits were weighed by a balance.
Method used for studying the physiological characters:
For this study, the mature fruits were collected randomly from the selected plants. Ten of selected fruits were put on the laboratory desk at a room temperature for recording their physical characteristics. The data were recorded at full ripe stage.
Shape of fruit:
It was determined by using a standard chart (Hossain and Ahmed, 1994) and eye estimation.
External appearance:
Appearance was determined by eye estimation and expressed in language.
Peeling quality:
Skin thickness was determined by eye estimation after peeling off the fruits.
Fruit weight:
After ripening, 10 fruits from each replication were taken for recording weight. Average weight of the ripe fruits was taken with the help of a triple beam balance where fraction of a gram could be read accurately.
Length, breadth and thickness of fruits:
Average diameter (cm) of the fruits in three different sides (Length, breadth, and thickness) was recorded to assess the dimension. Polar diameter was considered as the length of the fruit. The broad wish transverse diameter was taken as the breadth and the narrow wish diameter was the thickness. The diameters were taken with the help of slide calipers from the previously selected 10 fruits collected from each of the replicated variety.
Per cent peel and stone:
Peeling of the fruits and separation of the pulp from the stone were carefully done by hand. Weights of the peel and stone of the selected mango fruits were taken with the help of triple beam balance and percentage of peel and stone were also computed.
Length, breadth and thickness of stones:
The average length, breadth and thickness of stones for each replication were measured by a slide calipers and were recorded in cm.
Per cent edible and non-edible portion:
Percent edible and non-edible portions were calculated by the following formula:
Fresh fruit weight – (Stone weight + Peel weight)
Percent edible portion =
Fresh fruit weight
Percent non-edible portion = Peel (%) + Stone (%)
Peel to pulp ratio
It was recorded as the ration of pulp and peel weight
Pulp to stone ratio
It was calculated from the data obtained for stone weight dividing by the data obtained for pulp weight.
Isozyme analysis using polyacrylamide gel electrophoresis (PAGE):
Electrophoresis is the most useful means to separate protein polypeptides having different size and charge. Recently, polyacrylamide gel electrophoresis is most popular as a supporting matrix for electrophoresis while many supporting materials have been used. Vertical polyacrylamide gel electrophoresis (PAGE) was used for isozyme analysis from immature leaves of mango. The procedures for plant material preparation, sample extraction, gel preparation, application of the sample to the gel and gel staining were as follows.
Apparatus:
- Electrophoresis unit
- Power unit
- Refrigerator
- Gel dryer
- Centrifuge etc.
Stock solution:
Stock solution for gel (for 7% gel)
Sol. A: Acrylamide (29.2 g) and Bis acrylamide (0.8 g) was dissolved in about 20 ml of distilled water and the volume was made to 100 ml.
Sol. B: Tris (18.17 g) was dissolved in about 20 ml of distilled water and pH was adjusted at 8.8 and finally the volume was made to 100 ml by adding distilled water.
Sol. C: Tris (6.06 g) was dissolved in about 20 ml of distilled water and pH was adjusted at 6.06 and finally the volume was made to 100 ml by adding distilled water.
Sol. D (APS): Ammonium persulfate (0.1 g) was dissolved in 1 ml of distilled water.
Acrylamide and Bis was potent neurotoxins and absorbed through the skin. Therefore gloves and mask should be used during weighting and handling acrylamide and Bis.
Extraction buffer:
Tris (0.24 g) and 5.0 g of sucrose were dissolved in 80 ml of distilled water and the volume was made to 100 ml.
Electrode buffer:
Tris (1.2 g) and 5.8 g Glycine were dissolved in about 150 ml of distilled water, and the volume was made to 200 ml (diluted 10 times when used).
Tracking stain:
Bromophenole Blue (BPB) 0.1%
Bromophenole Blue (100 g) was dissolved in 80 ml distilled water, and the volume was made to 100 ml.
Staining solutions
POD – 1: 3 – Amino – 9 ethylcarbazole (1.05 g) and β – Napthol (0.725 g) was mixed and 500 ml Acetone was added to make the volume.
POD – 2: Hydrogen peroxide (20 ml) was added in 200 ml of water.
B POD: Tris (1.51 g) and 1.62 ml of acetic acid were dissolved in 800 ml distilled water and the volume was made to 1000 ml.
Gel preparation:
- The glass plates were wiped with 70% ethyl alcohol.
- The notched glass plate was placed with its spaces upside. The gasket (silicone rubber) was put on the glass plate, with its rising inner edges upside. The top ends of the gasket and the glass plate were matched.
- The plain glass plate was put on the notched glass plate.
- The side – edges of the matched glass plates was fixed by 2 clips. The clips were fixed in such way that it tightened.
- The separation gel (4.5%) was prepared by following the procedure in order
Stock solution | Quantity (2 gels) |
Sol. A | 18 ml |
Sol. B | 13.5 ml |
Sol. D (APS) | 27.30 µl (4 times) |
TEMED | 20 µl (4 times) |
- Separation gel solution (prepared freshly) was poured into the glass plate to the level of about 5 mm lower from the comb to be placed.
- A small amount of water or ethanol was added on top of the newly poured separation gel solution carefully to cut air. The surface of the gel was not disturbed.
- The solution was allowed to form gel for let 30-60 minutes or until a clear line between the gel and water seen.
- The top of the separation gel was washed with a small amount of water. Then the excess acrylamide and water were removed using filter paper.
10. The stacking gel was prepared using the following order.
Stock solution | Quantity (2 gels) |
Sol. A | 3.0 ml |
Sol. C | 3.0 ml |
Sol. D | 1.0 ml |
Distilled water | 8 ml |
TEMED | 2.5µl (2 times) |
11. The stacking gel solution was poured on top of the solid separation gel and the comb was inserted immediately between the glass plates carefully and allowed to stay there for 20-30 minutes.
12. The comb was then removed by pushing it up. The wells were washed with small amount of electrode buffer to remove any unpolymarized acrylamide.
Enzyme extraction:
- Mango leaf (0.1 g) was put into a mortar with a small amount of sea sand as crushing agent.
- Extraction buffer (1 ml) was added and the sample was crushed.
- The sample was crushed using a glass rod.
- Then the sample was kept into centrifuge machine to centrifuge at 14,000 rpm, 4°C for 10 minutes.
- The sample was kept in ice until use.
Loading sample:
- Electrode buffer solution was poured in a cylinder which was 100 ml volume and diluted 10 times. This solution was poured into the lower buffer tank of the vessel.
- Each glass plate was labeled. The clips and the gasket was removed
- Each glass plate was set with its notched glass plate facing the upper buffer tank using the pressure plate. Any bubble trapped at the bottom of the gel between the glass plates was removed carefully.
- The electrode buffer solution was poured into the upper buffer tank. 3 drop of marker stain was added.
- The prepared samples were injected to the cell chamber of stacking gel using a microsyringe.
Running:
- The lid was placed on the vessel.
- The vessel was connected to the power supply
- Constant current of 15 mA per plate (30 mA per vessel) was applied for 1.5 hr or when the BPB marker stain reached the bottom of the separation gel.
- After electrophoresis, power supply was turned off, the power leads were disconnected and the lid was removed.
Staining:
- The glass plates were taken off gently from the vessel. One of the glass plates was lifted using a spatula.
- The stacking gel was removed and the side-edges of the gel was cut off along the inner edges of the side-spacers. The orientation of the gel was marked by cutting a corner of it.
- The separation gel was removed from the glass plate onto the staining solution.
- The gel was stained following the procedure for specific enzyme.
Peroxidaze (POD):
- POD-1 of 60 ml was added to 240 ml of B-POD and filtrated.
- POD-2 (2 to 3 drops) was poured over the gel with dropper.
- The gel was incubated and shaked in the staining solution until bands appear at room temperature.
- After staining, the gel was washed with distilled water gently.
Isozyme analysis:
Calculation of Rf values
Electrophioretic mobilities of stained isozyme bands were calculated by the following formula:
Rf value
X = the distance from the origin to the leading edge of the isozyme band
Y = the distance traveled by the bromophenol blue.
Data analysis:
The analysis of isozyme data were performed using POPGENE, (version 1.32) (Yeh et al., 1999) and G-Stat, version 3.1 (Siegismund, 1995) computer program. When the most common (major) allele existed in a frequency less than or equal to 0.95 at a given locus, the locus was regarded as polymorphic. The percentage of polymorphic loci was determined by the help of gene stat program. Genetic distance values (D) (Nei, 1972) were calculated as
RESULTS AND DISCUSSION:
The results on 60 different characters of 20 mango germplasm have been presented and discussed under following heads. The summary of analysis of variance for all the parameters studied have been presented in Appdices II – VIII.
Studies on the morphological characteristics of 20 mango germplasm under Mymensingh conditions
The results of the study on morphological characteristics of different mango germplasm are presented in Table 2-11 and Plates 1 – 16. The findings have been discussed in this chapter under the following sub-headings.
Leaf Characteristics:
Results of different leaf characteristics of 20 mango germplasm are presented in Table 2 and Plates 1 & 2 and discussed below:
Length of leaf:
Significant variation was observed among different mango germplasm in respect of leaf length (Table 2). The leaf length of different mango varieties varied from 24.92 cm to 14.87. The highest length of leaf (24.92 cm) was found in the variety MI-008 followed by MI-025 (24.61 cm) which was statistically similar. The lowest leaf length (14.86 cm) was found in the variety MI-023.
Breadth of leaf:
Highly significant variation was observed among different mango germplasm in respect of leaf breadth (Table 2). Breadth of leaf ranged from 4.19 to 6.67 cm. MI-026 had the widest (6.67 cm) leaf breadth, which was statistically similar to MI-024 (6.53 cm), MI-008 (6.39 cm) and MI-030 (6.37 cm). The least leaf breadth (4.19 cm) was found in the accession MI-019. Hossain and Uddin (1995) reported that length and breadth of leaf in different mango varieties ranged from 16.75 to 24.70 cm and 3.76 to 8.30 cm, respectively.
Length of petiole:
A significant difference in respect of petiole length was observed in different mango germplasm. Length of petiole ranged from 1.67 to 4.53 cm. MI-019 had the longest (4.53 cm) petiole which was statistically similar to MI-047 (4.41 cm). The shortest petiole (1.67 cm) was found in MI-016.
Leaf shape:
The leaves of MI-016 and MI-024 were oblong-lanceolate in shape whereas MI-001, MI-019, MI-023 and MI-026 had the Elliptic lanceolate. The remaining germplasm had lanceolate leaves.
Leaf margin:
The leaf margin of MI-016, MI-019, MI-025, MI-030, MI-044 and MI-045 were flat, while the remaining germplasm had wavy leaf margin.
Leaf tip:
The leaf tip of MI-024 and MI-047 were obtuse whereas MI-001, MI-008, MI-020 and MI-025 had the acuminate. The remaining germplasm possessed acute type.
Table 2. Different leaf characteristics of 20 mango germplasm
| Germplasm | Length of leaf (cm) | Breadth of leaf (cm) | Length of petiole (cm) | Shape of leaf | Margin of leaf | Tip of leaf |
| MI-001 | 18.88 d-f | 4.98 d-f | 2.10 j | Elliptic lenceolate | Wavy | Acuminate |
| MI-002 | 19.27 d-f | 5.20 cd | 2.71gh | Lenceolate | Wavy | Acute |
| MI-004 | 20.20 c-e | 5.08 c-e | 2.65 h | Lenceolate | Wavy | Acute |
| MI-008 | 24.91 a | 6.39 a | 4.41 a | Lenceolate | Wavy | Acuminate |
| MI-009 | 21.71 bc | 4.82 d-g | 3.41 e | Elliptic lenceolate | Wavy | Acute |
| MI-016 | 19.06 d-f | 4.67 e-g | 1.67 k | Oblong lenceolate | Flat | Acute |
| MI-019 | 18.59 ef | 4.19 g | 2.90 g | Lenceolate | Wavy | Acute |
| MI-020 | 19.40 d-f | 4.52 fg | 3.23 ef | Elliptic lenceolate | Wavy | Acuminate |
| MI-022 | 18.26 f | 4.53 fg | 2.75 gh | Lenceolate | Wavy | Acute |
| MI-023 | 14.86 g | 4.38 g | 3.68 d | Elliptic lenceolate | Wavy | Acute |
| MI-024 | 21.28 bc | 6.53 a | 3.50 de | Oblong lenceolate | Wavy | Obtuse |
| MI-025 | 24.61 a | 5.45 bc | 3.91 c | Lenceolate | Flat | Acuminate |
| MI-026 | 20.45 b-d | 6.67 a | 3.57 de | Elliptic lenceolate | Wavy | Acute |
| MI-028 | 21.16 bc | 4.35 g | 3.14 f | Lenceolate | Wavy | Acute |
| MI-030 | 21.11 bc | 6.37 a | 3.54 de | Lenceolate | Flat | Acute |
| MI-043 | 21.05 bc | 5.49 bc | 4.16 b | Lenceolate | Wavy | Acute |
| MI-044 | 22.03 b | 5.69 b | 2.31 i | Lenceolate | Flat | Acute |
| MI-045 | 18.93 d-f | 5.19 cd | 3.73 d | Lenceolate | Flat | Acute |
| MI-047 | 18.72 ef | 5.20 cd | 4.53 a | Lenceolate | Wavy | Obtuse |
| MI-048 | 17.99 f | 4.61 fg | 3.71 d | Lenceolate | Wavy | Acute |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
|
Flowering and some other related characters of 20 mango germplasm:
The flowering and some other related characters of 20 mango germplasm are presented in Table 3 and discussed below.
Time of flower bud emergence:
The time of flower bud emergence in all the germplasm under study took place between December 05 to January 12 (Table 3). Flower bud emergence was the earliest (December 05) in the germplasm MI-019, which was closely followed by MI-023 (December 06). Germplasm MI-008 was the latest (January 12) in flower bud emergence. The other germplasm were in intermediary position. The present investigation in partial agreement with the research findings of Haque et al. (1993) who evaluated 20 mango cultivars at Southern Bangladesh and reported that the flower bud emergence took place from third week of December to last week of January.
Time of first panicle emergence:
The panicle was first noticed in MI-019 (December, 30) followed by MI-020 (January, 05) while MI-008 was late (February, 06). This result partially supported the findings of Uddin et al. (1997) who evaluated 14 mango germplasm at Chapai Nawabganj and reported that the panicle emergence took place from December, 25 to February, 23.
Time of first flower opening:
The time of first flower opening in all the germplasm under the study took place between January, 18 to February, 22 (Table 3). Flower were opened first in MI-019 (January, 18) and MI-008 was the latest (February, 22). The other germplasm were intermediary in position. The present result partially supported the findings of Uddin et al. (1997) who reported that first flowering took place between January 25 to March 10. Majumder and Sharma (1990) reported the flowering time varied with the varieties and area where they grown. Bose (1985) also reported the same observation.
Time of full bloom:
Full blooming was first noted from February 05 in MI-019 and last on March 17 in MI-004. The other germplasm were intermediary in position. Uddin et al. (1997) reported that first full blooming appeared in February 26 and last in March 19. The variability found in the present study is in agreement with the findings of Valmayor (1962) who reported that the variation of blooming period is dependent upon a combination of environmental factors and the condition of the plant.
Date of harvest:
The fruits of all the germplasm were harvested between June 05 and July 06, 2005 (Table 3). MI-023 and MI-022 were the earliest and latest, respectively in the harvesting time. Remaining germplasms were between the two ends. The results are in agreement with the findings of Bhuyan and Islam (1989) and Sardar and Hossain (1993). But Uddin et al. (1997) evaluats 14 mango cultivars at Chapai Nawabganj reported that the fruits of all the germplasm were harvested between June, 20 to July, 15.
Days to maturity (from flowering to harvest):
A wide variation was observed among the germplasm in respect of days to maturity. Maximum time (169.00 days) was required for maturity in MI-019, which was significantly different in comparison to other germplasm. MI-023 took minimum time (115.00 days) closely followed by MI-026 (117.00 days), MI-030 (119.67 days) and MI-002 (121.33 days). No variation was observed between MI-028 (139.33 days) and MI-048 (138.67 days); MI-047 (136.33 days); MI-004 (136.00 days) and MI-016 (136.00 days). These results were found to be similar to the observation of Hossain (1989) who reported that mango under Bangladesh conditions it took about four to six months to reach maturity after flowering. These findings differed with that of Sardar et al. (1998) who consented that harvesting time varied from 92-134 days in some popular mango cultivars under the climatic conditions of Rajshahi. This might be environmental fluctuation over the year, variety and the locality.
Table 3. Flowering and some other related characters of 20 mango germplasm
| Germplasm | Time of flower bud emergence | Time of first panicle emergence | Time of first flower opening | Time of full bloom | Date of harvest | Days of maturity (from flowering to harvest) |
| MI-001 | December, 26 | February, 02 | February, 20 | March, 13 | 26/6/2005 | 129.67 h |
| MI-002 | December, 28 | January, 30 | February, 21 | March, 12 | 19/6/2005 | 121.33 j |
| MI-004 | December, 29 | January, 31 | February, 19 | March, 17 | 03/7/2005 | 136.00 f |
| MI-008 | January, 12 | February, 06 | February, 22 | March, 16 | 27/6/2005 | 127.00 i |
| MI-009 | December, 27 | January, 26 | February, 18 | March, 09 | 29/6/2005 | 133.00 g |
| MI-016 | December, 25 | January, 29 | February, 19 | March, 10 | 03/7/2005 | 136.00 f |
| MI-019 | December, 05 | December, 30 | January, 18 | February, 05 | 05/7/2005 | 169.00 a |
| MI-020 | December, 12 | January, 05 | January, 20 | February, 09 | 04/7/2005 | 167.33 b |
| MI-022 | December, 24 | January, 28 | February, 20 | March, 12 | 06/7/2005 | 141.00 d |
| MI-023 | December, 06 | January, 15 | February, 12 | March, 05 | 05/6/2005 | 115.00 m |
| MI-024 | December, 29 | January, 23 | February, 15 | March, 02 | 30/6/2005 | 138.00 e |
| MI-025 | December, 30 | January, 30 | February, 17 | March, 08 | 26/6/2005 | 130.67 h |
| MI-026 | December, 28 | January, 26 | February, 20 | March, 11 | 16/6/2005 | 117.00 l |
| MI-028 | December, 27 | January, 24 | February, 14 | March, 13 | 30/6/2005 | 139.33 e |
| MI-030 | December, 15 | January, 20 | February, 13 | March, 09 | 10/6/2005 | 119.67 k |
| MI-043 | December, 20 | January, 25 | February, 20 | March, 13 | 28/6/2005 | 130.67 h |
| MI-044 | December, 30 | January, 26 | February, 19 | March, 13 | 25/6/2005 | 128.67 hi |
| MI-045 | December, 20 | January, 13 | January, 30 | March, 16 | 25/6/2005 | 147.33 c |
| MI-047 | December, 28 | January, 30 | February, 20 | March, 10 | 02/7/2005 | 136.33 f |
| MI-048 | December, 23 | January, 26 | February, 16 | March, 08 | 02/7/2005 | 138.67 e |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Different flower characteristics:
The flower characteristics in all the germplasm under the study are presented in Table 4 and discussed below:
Leafy bracts:
Leafy bracts were present in most of the germplasm under study (Table 4) except in MI-030 and MI-047.
Flower diameter:
A significant difference in flower diameter was observed in different germplasm studied (Table 4). The maximum flower diameter was recorded in MI-016 (6.90 mm), MI-009 (6.80 mm) and MI-004 (6.80 mm) while the minimum was recorded in MI-025 (4.00 mm) and MI-008 (4.50 mm).
Type of flower:
The flower type of 20 mango germplasm under study was pentamerous except MI-024 and MI-030 (Tetra and Pentamerous) (Table 4).
Nature of disc:
The nature of disc in all the germplasm were swollen (Table 4).
Number of stamens:
The number of stamens of 20 mango germplasm varied from 1 to 3 (Table 4). The maximum number of stamens was recorded in MI-020 (03) while minimum was in all the germplasm except MI-022 (02).
Density of flower:
Scarcely flower was observed in all the mango germplasm under study (Table 4) and densely flower was observed in MI-030 and MI-047.
Table 4. Different flower characteristics of 20 mango germplasm
| Germplasm | Leafy bracts | Flower diameter (mm) | Type of flower | Nature of disc | Number of stamen | Density of flower |
| MI-001 | Present | 4.90 c | Pentamerous | Swollen | 1 | Scarcely |
| MI-002 | Present | 6.00 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-004 | Present | 6.80 a | Pentamerous | Swollen | 1 | Scarcely |
| MI-008 | Present | 4.50 d | Pentamerous | Swollen | 1 | Scarcely |
| MI-009 | Present | 6.80 a | Pentamerous | Swollen | 1 | Scarcely |
| MI-016 | Present | 6.90 a | Pentamerous | Swollen | 1 | Scarcely |
| MI-019 | Present | 6.00 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-020 | Present | 5.90 b | Pentamerous | Swollen | 3 | Scarcely |
| MI-022 | Present | 6.10 b | Pentamerous | Swollen | 2 | Scarcely |
| MI-023 | Present | 6.00 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-024 | Present | 6.10 b | Tetra & Pentamerous | Swollen | 1 | Scarcely |
| MI-025 | Present | 4.00 e | Pentamerous | Swollen | 1 | Scarcely |
| MI-026 | Present | 5.80 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-028 | Present | 5.90 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-030 | Absent | 6.00 b | Tetra & Pentamerous | Swollen | 1 | Densely |
| MI-043 | Present | 6.00 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-044 | Present | 6.00 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-045 | Present | 6.10 b | Pentamerous | Swollen | 1 | Scarcely |
| MI-047 | Absent | 6.00 b | Pentamerous | Swollen | 1 | Densely |
| MI-048 | Present | 6.10 b | Pentamerous | Swollen | 1 | Scarcely |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Panicle characteristics:
Results of different panicle characteristics of 20 mango germplasm are presented in Table 5 and Plates 3 – 6 and discussed below:
Panicle color:
The panicle color in most of the varieties varied from light green to dark red (Table 5). Panicle color of MI-002, MI-009, MI-016, MI-024, MI-025 and MI-048 was light green while MI-004, MI-019, MI-022, MI-023, MI-043 and MI-044 had dark red colored panicle. MI-008 had light green with red patches while MI-026, MI-030 and MI-045 had green colored panicle with red patches. Crimson colored panicle was observed in MI-020 but the rest were light red colored panicle.
Panicle position:
Most of the panicle position of 20 mango germplasm were both terminal and axillary and remaining were terminal which was found in MI-023, MI-024, MI-026, MI-043 and MI-047 (Table 5).
Panicle shape:
The panicle of MI-001, MI-008, MI-016, MI-023, MI-030, MI-047 and MI-048 were conical in shape whereas MI-004, MI-024, MI-025, MI-026 and MI-044 had the pyramidal in shape. The remaining germplasms were broadly pyramidal type (Table 5).
Branching habit:
Most of the mango germplasm had secondary branches in their panicle except MI-016 and MI-045 which had tertiary branches as well. MI-009 and MI-028 had both secondary and tertiary branches.
Table 5. Panicle characteristics of 20 mango germplasm at GP center
| Germplasm | Color | Position | Shape | Branching habit |
| MI-001 | Light red | Terminal and axillary | Conical | Secondary |
| MI-002 | Light green | Terminal and axillary | Broadly pyramidal | Secondary |
| MI-004 | Dark red | Terminal and axillary | Pyramidal | Secondary |
| MI-008 | Light green with red patches | Terminal and axillary | Conical | Secondary |
| MI-009 | Light green | Terminal and axillary | Broadly pyramidal | Secondary and tertiary |
| MI-016 | Light green | Terminal and axillary | Conical | Tertiary |
| MI-019 | Dark red | Terminal and axillary | Broadly pyramidal | Secondary |
| MI-020 | Crimson | Terminal and axillary | Broadly pyramidal | Secondary |
| MI-022 | Dark red | Terminal and axillary | Broadly pyramidal | Secondary |
| MI-023 | Dark red | Terminal | Conical | Secondary |
| MI-024 | Light green | Terminal | Pyramidal | Secondary |
| MI-025 | Light green | Terminal and axillary | Pyramidal | Secondary |
| MI-026 | Green with red patches | Terminal | Pyramidal | Secondary |
| MI-028 | Light red | Terminal and axillary | Broadly pyramidal | Secondary and tertiary |
| MI-030 | Green with red patches | Terminal and axillary | Conical | Secondary |
| MI-043 | Dark red | Terminal | Broadly pyramidal | Secondary |
| MI-044 | Dark red | Terminal and axillary | Pyramidal | Secondary |
| MI-045 | Green with red patches | Terminal and axillary | Broadly pyramidal | Tertiary |
| MI-047 | Light red | Terminal | Conical | Secondary |
| MI-048 | Light green | Terminal and axillary | Conical | Secondary |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
1 Number of panicles/shoot:
Highly significant difference in respect of number of panicles/shoot was observed in all the mango germplasm studied (Table 6). The maximum number of panicles per shoot was recorded in MI-022 (3.57) followed by MI-028 (3.32) and MI-019 (3.09) while the minimum number of the panicles (1.10) was recorded in MI-043 closely followed by MI-023 (1.23), MI-047 (1.31) and MI-030 (1.41). Bhuyan and Islam (1989), Haque et al. (1993) and Islam et al. (1995) also observed the variation in number of panicles per shoot among different mango varieties.
Length of panicle:
Highly significant variation was observed among different mango germplasm in respect of panicle length (Table 6). Length of panicle ranged from 19.46 to 36.66 cm. MI-045 had the longest (36.66 cm) panicle length. Significant variation was observed among most of the tested germplasms. The shortest panicle (19.46 cm) was found in the accession MI-002, which was statistically different from the remaining germplasms. Hossain and Talukdar (1974) reported that panicle length in different mango varieties ranged from 13.97 to 22.60 cm, but Islam et al. (1995) found 27.79 to 33.77 cm in eight mango varieties. Reza et al. (1995) evaluated 18 exotic mango germplasms at Chapai Nawabganj and reported that the length of panicle ranged from 21.10 to 40.90 cm.
Breadth of panicle:
Panicle breadth was statistically varied among the tested germplasm (Table 6). The maximum panicle breadth (22.69 cm) was observed in MI-043 followed by MI-020 (22.19 cm). Minimum breadth was recorded in MI-002 (11.17 cm). Hossain and Ahmed (1994) also recorded the variation in panicle breadth among the varieties studied.
Number of main branches per panicle:
The number of main branches per panicle ranged from 46.26 to 23.52 (Table 6). The germplasm MI-045 had the highest number (46.26). On the contrary, MI-002 had the lowest (23.52) number of main branches, which was closely followed by MI-001 (24.29). The present result is partial accordance with the findings of Haque et al. (1993) who recorded 10 to 74 number of main branches per panicle in 20 mango cultivars. The results have also some similarities with the findings of Islam et al. (1995).
Table 6. Some other related panicle characteristics of 20 mango germplasm
| Germplasm | Number of panicles/shoot | Length of panicle (cm) | Breadth of panicle (cm) | No. of main branches/panicle |
| MI-001 | 1.63 e | 24.31 h | 11.62 k | 24.29 n |
| MI-002 | 2.61 c | 19.46 m | 11.17 l | 23.52 o |
| MI-004 | 1.60 ef | 27.30 e | 14.08 h | 26.82 l |
| MI-008 | 1.69 e | 25.58 g | 12.85 j | 29.35 h |
| MI-009 | 2.54 c | 22.66 j | 14.55 g | 42.73 c |
| MI-016 | 2.06 d | 21.90 k | 12.56 j | 30.25 g |
| MI-019 | 3.09 b | 21.24 l | 20.46 d | 30.60 g |
| MI-020 | 2.13 d | 28.74 d | 22.19 b | 45.48 b |
| MI-022 | 3.57 a | 27.80 e | 21.34 c | 33.52 f |
| MI-023 | 1.23 gh | 21.53 kl | 12.64 j | 25.45 m |
| MI-024 | 1.68 e | 23.79 hi | 14.22 gh | 29.56 h |
| MI-025 | 1.55 ef | 29.21 d | 16.46 f | 28.42 k |
| MI-026 | 2.10 d | 27.54 e | 13.63 i | 28.65 i-k |
| MI-028 | 3.32 ab | 27.37 e | 20.37 d | 34.41 e |
| MI-030 | 1.41 e-g | 26.33 f | 13.92 hi | 29.25 hi |
| MI-043 | 1.10 h | 32.51 c | 22.69 a | 36.48 d |
| MI-044 | 2.44 c | 35.36 b | 20.54 d | 30.50 g |
| MI-045 | 2.30 cd | 36.66 a | 18.46 e | 46.26 a |
| MI-047 | 1.31 f-h | 23.55 i | 11.40 kl | 29.17 h-j |
| MI-048 | 1.67 e | 26.18 f | 14.53 g | 28.57 jk |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Other flower characteristics:
Other flower characteristics of 20 mango germplasm percentage of male, bisexual and unopened flower, number of fruit set/panicle and fruit harvested per panicle are presented in Table 7 and discussed below:
Male flower per panicle (%):
The result revealed that the male flower (%) of different mango germplasm was varied significantly from 93.48 to 56.37 (Table 7). The highest male flower (93.48%) was obtained from the germplasm MI-002, which was statistically similar to those of MI-009 (93.16%), MI-030 (92.91%) and MI-026 (92.70%). The lowest male flower (56.37%) was recorded form MI-025 followed by MI-044 (58.45%) which was statistically similar. Hossain and Talukdar (1974) found 29.00% to 59.91% male flower in 38 mango varieties. Iqbal et al. (1995) observed 69.86% to 93.39% male flower in 18 mango germplasm.
Bisexual flower per panicle (%):
Significant variation was found in case of bisexual flower (%) of the germplasm under study and the percentage varied from 36.08 to 1.73 (Table 7). MI-025 had the highest percentage (36.08%) of bisexual flowers. Minimum bisexual flower (1.73%) per panicle was noted in MI-002 which was statistically similar with that of MI-030 (1.86%). The results of the present experiment is in partial agreement with the findings of Singh (1978) who reported that in South India mangoes it varied from 16.41% in Neelum to 3.17% in Alampur Beneshan. Maiti et al. (1971) asserted that number of bisexual flowers varied with variety and season. Uddin et al. (1995) who evaluated 18 exotic mango germplasm at Chapai Nawabganj and reported that the bisexual flower of the studied germplasm varied from 6.61 to 30.46%.
Unopened flower per panicle:
A wide variation was observed among the tested germplasm in respect of unopened flower (%). Maximum unopened flowers (19.77%) was recorded in MI-044 (Table 7), which differed significantly from the remaining germplasms. Minimum number of unopened flowers (2.29%) was observed in MI-002, which was closely followed by MI-043 (2.57%). This finding differs from that of Hossain and Talukder (1974), who recorded 35.50% to 84.66% unidentified flower. This might have occurred due to the variation in environmental factors.
Number of initial fruit set per panicle:
Fruit set per panicle was the highest (30.50) in MI-019 (Table 7) and lowest (5.15) in MI-008. Iqbal et al. (1995) found 1.22 to 37.53 fruit set per panicle in 18 mango germplasm. Singh (1978) stated that fruit set is varietal character also depends upon time of flowering, efficient cross-pollination and fruit drop intensity.
Number of final fruit set per panicle:
A significant difference in respect of number of final fruit set per panicle was observed in different mango germplasm studied (Table 7). The highest number of final set was (5.98) in MI-028 while MI-043 (1.20), MI-044 (1.22), MI-008 (1.23) and MI-030 (1.28) showed the lowest which were statistically similar.
Fruit harvested per panicle (%):
Wide variation was observed among the mango germplasm in case of fruit harvest per panicle (Table 7). Maximum number was recorded in MI-047 (36.47%) and minimum number of fruits was harvested in MI-044 (4.58%). From an experiment Hossain and Talukdar (1974) obtained 0.17% to 7.54% fruit harvest per panicle in different mango varieties. In the present study, variation in fruit harvest had been observed. Such variation might have occurred due to varied differences occurring under different ecological factors.
Table 7. Different types of flower, number of fruit set and fruit harvest per panicle in different germplasm of mango
| Germplasm | Male flowers/ |
panicle
(%)Bisexual flowers/
panicle
(%)Unopened flowers/
panicle
(%)Number of initial fruit set/panicleNumber of final fruit set/panicleFruit harvested/
panicle
(%)MI-00189.24 c6.46 f3.85 gh7.16 i1.53 fg21.41 cdMI-00293.48 a1.73 j2.29 i6.88 i1.83 ef26.41 bMI-00490.10 bc3.24 i4.41 e-g9.05 g1.33 fg16.29 fgMI-00891.19 b-c4.34 g-i4.61 ef5.15 j1.23 g20.55 cdMI-00992.90 a4.74 gh3.25 h23.30 e2.47 d11.49 hMI-01691.68 b3.54 hi4.60 ef6.77 i1.58 e-g22.36 cdMI-01985.52 e13.32 c3.53 h30.49 a3.38 c11.31 hMI-02089.13 c6.71 f3.52 h24.45 d3.45 c14.26 gMI-02282.71 e13.50 c4.31 e-g27.62 c4.35 b19.69 deMI-02389.21 c7.94 e3.50 h14.41 f2.05 de15.23 fgMI-02478.41 f9.47 d11.33 c9.10 g2.48 d26.25 bMI-02556.37 h36.08 a4.82 e8.58 gh1.50 fg10.91 hMI-02692.70 ab3.72 h3.37 h8.66 g1.61 e-g21.48 cdMI-02886.07 d10.69 d4.56 e-g24.86 d5.98 a23.43 cMI-03093.16 a1.86 j4.52 e-g6.43 i1.28 g19.67 deMI-04392.49 b4.81 gh2.57 i7.47 hi1.20 g17.13 efMI-04458.45 h21.63 b19.76 a29.02 b1.22 g4.58 jMI-04571.43 g14.06 c14.20 b25.30 d1.56 e-g7.59 iMI-04786.04 d9.85 d3.91 f-h6.64 i2.29 d36.47 aMI-04886.66 d5.45 fg8.26 d6.61 i1.64 e-g26.33 b
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Physical characteristics of the fruits of 20 mango germplasm:
Physical characteristics of mango are presented in Table 8, Plates 7 – 16 and discussed under the following sub-headings:
Qualitative characteristics of fruits:
Results of different qualitative characters of fruits of 20 mango germplasm are presented in Table 8 and discussed below:
Shape of fruits:
The fruit shape of MI-004, MI-0047 and MI-048 were oblong oval but that of MI-002 and MI-024 were oblongish oval. MI-001 and MI-022 were ellipsoid and MI-019, MI-026 and MI-045 were almost roundish and MI-030 was round. The shape of MI-043 and MI-009 were oblong whereas MI-008, MI-020 and MI-023 were oblique oblong. MI-025 was ovalish oblong and germplasm MI-028 was oblong obliqe. Whereas MI-016 was obliquely oval. The results are in conformity with the findings of Saha and Hossain (1988) and Ghose and Hossain (1988).
External appearances:
Good appearance of mango has the highest phenotypic acceptability to the consumers. Among the 20 mango germplasm appearance of MI-002, MI-004, MI-022, MI-023, MI-028, MI-030, MI-044 and MI-045 were good. Fruits of MI-001, MI-008, MI-009, MI-019, MI-024, MI-025, MI-026, MI-043, MI-047 and MI-048 were medium. On the contrary, MI-016 and MI-020 were in poor appearance.
Skin color at ripe stage:
The skin color of ripe fruits of MI-004, MI-008 and MI-019 were greenish yellow while MI-001, MI-022, MI-023, MI-026, MI-028, MI-047 and MI-048 were yellowish green. Ripe fruits of MI-024 and MI-025 were light green while that of MI-016 and MI-020 were green. Germplasm MI-044 was reddish green whereas MI-002, MI-009, MI-030 and MI-043 had yellow colored fruits. The variability found in the present study conform the findings of Mukherjee (1997), who reported that fruit color at maturity was dependent on genotype.
Peeling quality:
Peeling was easy in MI-001, MI-002, MI-004, MI-008, MI-009, MI-022, MI-023, MI-024, MI-025, MI-026, MI-028, MI-030, MI-043, MI-047, and MI-048. But it as difficult in the remaining germplasm (Table 8).
Skin thickness:
The fruit skin was thick in MI-016, MI-044 and MI-045 while it was thin in MI-001, MI-019, MI-022, MI-025, MI-028, MI-030, MI-043 and MI-048. Fruits were medium skinned in the remaining germplasm (Table 8).
Pulp color:
The pulp color of the fruits were orange in MI-001, MI-004, MI-023 and MI-028; light yellow in MI-044 and MI-045; dark yellow in MI-048 and cream yellow in MI-016 and MI-024. Germplasm MI-002, MI-008, MI-009, MI-019, MI-020, MI-022, MI-025, MI-026, MI-030, MI-043 and MI-047 had the yellow pulp color (Table 8).
Yield and fruit characteristics:
Results of yield and different characteristics of fruits of 20 mango germplasm are presented in Table 9 and discussed below:
Fruit weight:
Highly significant variation was found in the fruit weight of different mango germplasm (Table 9). The heaviest fruit (534.56 g) was recorded in MI-016. The lightest fruit was obtained from MI-043 (178.44 g) and other germplasms were intermediary position. In the present study variation in fruit weight of the different mango germplasm had been noticed. This variation may be due to genetic or physiological factors. Lodh et al. (1974), Haque et al. (1993) and Iqbal et al. (1995) also reported the variation of fruit weight among the different mango varieties.
Fruit size:
Length, breadth and thickness of fruits were studied and the results are presented in Table 9.
Fruit length: A wide range of variation was observed among the germplasm in respect of fruit length. MI-044 produced the longest fruit (21.52 cm) whereas MI-030 produced the shortest fruit (7.86 cm).
Fruit breadth: A significant variation in respect of fruit breadth was found in all the mango germplasm studied (Table 9). MI-044 produced the widest fruit (10.71 cm) while the narrowest fruit was noticed in MI-043 (6.62 cm) followed by MI-030 (6.76 cm).
Fruit thickness: All the mango germplasm under study showed significant difference in fruit thickness. The thickest fruit (9.81 cm) was in MI-016 followed by MI-025 (9.69 cm), MI-004 (9.69 cm) and MI-009 (9.62 cm) which were statistically similar. The lowest thickness was found in MI-030 (5.72 cm) followed by MI-043 (5.98 cm) which were also statistically similar. From an experiment Sardar et al. (1998) reported that length, breadth and thickness of mango fruits varied from 7.6 to 15.5, 5.5 to 8.9 and 5.0 to 8.2 cm, respectively. Mollah and Siddique (1973), Prasad (1977) and Saha and Hossain (1988) also found different fruit size in different mango varieties.
Fruit yield per plant:
Fruit yield (Number): A significant difference in respect of fruit yield (number) was found in all the germplasm studied (Table 9). MI-009 produced the highest number of fruits per plant (54.33) while the lowest numbers was in MI-043 (6.67). MI-009 showed the superiority over the rest of the germplasms. Singh (1978) reported that, the start of bearing fruit number in the plant may be as low as 10 to 15 per tree rising to 50 to 75 fruits, depends on tree size in the subsequent year.
Fruit yield (weight): The fruit weight/tree in all the mango germplasm under study varied from 27.32 kg to 1.19 kg (Table 9). The highest yield of fruit was recorded in MI-009 (27.31 kg) while the lowest yield was in MI-043 (1.19 kg).
Table 8. Qualitative characteristics of 20 mango germplasm in GP center
| Germplasm | Shape of fruit | External appearance | Skin color at ripe stage | Peeling quality | Skin thickness | Pulp color |
| MI-001 | Ellipsoid | Medium | Yellowish green | Easy | Thin | Orange |
| MI-002 | Oblongish oval | Good | Yellow | Easy | Medium | Yellow |
| MI-004 | Oblong oval | Good | Greenish yellow | Easy | Medium | Orange |
| MI-008 | Oblique oblong | Medium | Greenish yellow | Easy | Medium | Yellow |
| MI-009 | Oblong | Medium | Yellow | Easy | Medium | Yellow |
| MI-016 | Obliquely oval | Poor | Green | Difficult | Thick | Cream yellow |
| MI-019 | Almost roundish | Medium | Greenish yellow | Difficult | Thin | Yellow |
| MI-020 | Oblique oblong | Poor | Green | Difficult | Medium | Yellow |
| MI-022 | Ellipsoid | Good | Yellowish green | Easy | Thin | Yellow |
| MI-023 | Oblique oblong | Good | Yellowish green | Easy | Medium | Orange |
| MI-024 | Oblongish oval | Medium | Light green | Easy | Medium | Cream yellow |
| MI-025 | Ovalish oblong | Medium | Light green | Easy | Thin | Yellow |
| MI-026 | Almost roundish | Medium | Yellowish green | Easy | Medium | Yellow |
| MI-028 | Oblong oblique | Good | Yellowish green | Easy | Thin | Orange |
| MI-030 | Round | Good | Yellow | Easy | Thin | Yellow |
| MI-043 | Oblong | Medium | Yellow | Easy | Thin | Yellow |
| MI-044 | Almost roundish | Good | Reddish green | Difficult | Thick | Light yellow |
| MI-045 | Almost roundish | Good | Greenish red | Difficult | Thick | Light yellow |
| MI-047 | Oblong oval | Medium | Yellowish green | Easy | Medium | Yellow |
| MI-048 | Oblong oval | Medium | Yellowish green | Easy | Thin | Dark yellow |
Note: Variety means having the same lettr are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Table 9. Yield and fruit characteristics of 20 mango germplasm
| Germplasm | Fruit weight (g) | Fruit size (cm) | Yield/plant | |||
| Length | Breadth | Thickness | Number | Weight (kg) | ||
| MI-001 | 263.44 k | 12.82 bc | 7.19 ef | 7.38 fg | 45.00 de | 11.85 d |
| MI-002 | 281.95 j | 10.22 ef | 8.67 cd | 8.71 b-e | 34.67 h | 9.77 e-g |
| MI-004 | 312.45 i | 13.17 b | 10.45 a | 9.69 a | 14.33 k | 4.47 k |
| MI-008 | 221.15 m | 11.23 de | 7.36 d-f | 7.53 fg | 41.67 f | 9.21 f-h |
| MI-009 | 502.83 c | 12.94 bc | 9.42 bc | 9.62 ab | 54.33 a | 27.31 a |
| MI-016 | 534.56 a | 13.18 b | 9.09 bc | 9.81 a | 10.33 m | 5.52 j |
| MI-019 | 520.72 b | 11.35 de | 9.59 bc | 8.45 b-f | 30.67 j | 15.97 c |
| MI-020 | 259.90 kl | 10.45 d-f | 8.53 c-e | 7.98 d-f | 44.00 de | 11.43 de |
| MI-022 | 322.47 i | 13.46 b | 9.27 bc | 8.22 c-f | 45.33 cd | 14.61 c |
| MI-023 | 182.31 op | 9.41 fg | 7.46 d-f | 7.46 fg | 46.67 c | 8.50 gh |
| MI-024 | 481.97 d | 13.38 b | 9.31 bc | 8.99 b-d | 43.33 e | 20.88 b |
| MI-025 | 365.33 g | 12.77 bc | 9.92 b | 9.69 a | 34.00 h | 12.42 d |
| MI-026 | 334.92 h | 11.04 de | 10.14 ab | 9.22 bc | 43.33 de | 14.51 c |
| MI-028 | 194.36 no | 11.79 cd | 7.58 d-f | 6.72 gh | 49.00 b | 9.52 d-f |
| MI-030 | 202.25 n | 7.86 h | 6.76 f | 5.72 h | 31.67 i | 6.40 ij |
| MI-043 | 178.44 p | 9.63 fg | 6.62 f | 5.98 h | 6.67 n | 1.19 l |
| MI-044 | 420.89 e | 21.52 a | 10.71 a | 8.48 b-f | 12.00 l | 5.05 jk |
| MI-045 | 388.55 f | 11.80 cd | 9.75 b | 8.93 b-d | 13.67 kl | 5.31 j |
| MI-047 | 250.22 l | 8.90 gh | 7.57 d-f | 7.72 e-g | 41.33 f | 10.34 d |
| MI-048 | 194.00 no | 11.63 cd | 7.72 d-f | 6.60 gh | 36.33 g | 7.04 hi |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Some general characteristics of fruits:
Edible portion (%), non-edible portion (%), peel weight (g), pulp weight (g) and peel to pulp ratio of 20 mango germplasm are presented in Table 10 and discussed below:
Per cent edible portion:
Percent edible portion of fruits is an important character for selecting quality fruits and in this study it significantly varied from 80.53% to 54.40% (Table 10). MI-024 had the highest edible portion followed by MI-019 (80.27%) and MI-009 (78.62%) and MI-004 had the lowest closely followed by MI-023 (57.04%), MI-048 (57.57%) and MI-008 (58.07%). This result agreed with the findings obtained by Saha and Hossain (1988), Bhuyan and Islam (1989) and Sardar et al. (1995).
Per cent non-edible portion:
Per cent peel:
Percent peel of different mango germplasm ranged from 26.65% to 8.93% (Table 10). MI-004 had maximum peel. The lowest percentage of peel (8.93) was observed in MI-024 followed by MI-019 (9.87). The result is some what in agreement with the findings of Bhuyan and Islam (1986) who found 9.92 to 17.31% peel from 13 mango varieties. The result has also some similarities with the findings of Ahmad et al. (1989) where they observed 11.70 to 20.50% peel in 10 mango varieties.
Per cent stone:
A highly significant difference in respect of percent stone was observed in all germplasm studied (Table 10). MI-048 contained the heaviest stone (21.33%) followed by MI-023 (20.38%) and MI-030 (20.31%) which were statistically similar. The lightest stone was found in MI-024 (8.60%) closely followed by MI-020 (8.72%) and MI-019 (9.42%). Bhuyan and Islam (1986) observed 8.07 to 19.25% stone portion in 13 mango varieties and Sardar et al. (1998) found 12.5 to 26.2% stone portion in 10 mango varieties.
Per cent total:
All the germplasm varied significantly in respect of percent total non-edible portion (Table 10). The highest percentage of total non-edible portion (44.72%) was obtained from MI-004 whereas the germplasm MI-019 had the lowest percentage of non-edible portion (19.29%). Remaining germplasms were between the two groups. The present result is in partial agreement with the research findings of Bhuyan and Islam (1986) who recorded 18.51 to 35.06% non-edible portion.
Stone characteristics:
The stone characteristics in respect of weight length, breadth, thickness and pulp to stone ratio were studied and the results are presented in Table 11.
Stone length: Stone length of different mango germplasm showed wide variations, MI-022 had the longest stone (11.16 cm) closely followed by MI-016 (10.80 cm) and MI-024 (10.69 cm) while MI-030 produced the smallest stone (6.87 cm) which was also closely followed by MI-023 (7.98 cm) which were statistically similar.
Stone breadth: The widest stone (6.11 cm) was also noted in MI-022, which was statistically similar to MI-004 (6.02 cm). No variation was observed among MI-016, MI-019 and MI-009 (Table 11). The narrowest stone (3.70 cm) was observed in MI-001 followed by MI-030 (4.37 cm) and MI-047 (4.39 cm).
Stone thickness: The stone thickness of different mango germplasm varied from 4.05 cm to 1.36 cm. Highest stone thickness was observed in MI-026 (4.05 cm). The germplasm MI-019 produced the thinnest stone (1.36 cm), which was significantly different in comparison of other germplasm. Stone characteristics like length, breadth and thickness were studied by Haque et al. (1993), Saha et al. (1995) and Sardar et al. (1998). From their findings it was observed that the different varieties maintained distinctive stone characteristics of their own. In the present studies, similar variation on the stone size was observed.
Fruit characteristics of mango germplasm MI-001 and MI-002
Fruit characteristics of mango germplasm MI-009 and MI-016
4.2.5 Peel weight
All the germplasm under study showed highly significant differences in peel weight (Table 10). Germplasm MI-004 had the maximum weight (88.24 g) followed by MI-016 (64.70 g) while minimum weight in MI-028 (34.32 g) closely followed by MI-030 (35.95 g) and MI-043 (36.11).
4.2.6 Pulp weight
Pulp weight of different germplasm showed wide variations (Table 10). MI-019 had the highest pulp weight (431.76 g) followed by MI-016 (416.76 g) whereas the minimum weight was found in MI-023 (106.59 g) closely followed by MI-048 (115.22 g) and MI-043 (155.38 g).
4.2.7 Peel and pulp ratio
Peel and pulp ratio significantly varied from 1.94 to 8.65 among different mango germplasm (Table 10). The highest ratio (8.65) was observed in MI-019, which was closely followed by MI-004 (1.94).
4.2.8 Pulp to stone ratio
Highly significant difference in respect of pulp to stone ratio was observed in all the mango germplasm studied (Table 11). Maximum pulp to stone ratio (0.38) was found in MI-023 while minimum ration (0.11) was recorded in MI-019 which was closely followed by MI-020 (Table 10). Other germplasm were between the two groups. These findings differ with that of Hossain and Talukdar (1974) who recorded ratio 0.05 to 0.44. This might have occurred due to the varietal differences and/or the variation of environmental factors.
Table 10. Some general fruits characteristics of 20 mango germplasm
| Germplasm | Edible portion (%) | Non-edible portion (%) | Peel weight (g) | Pulp weight (g) | Peel to pulp ratio | ||
| Peel | Stone | Total | |||||
| MI-001 | 61.62 h | 19.82 cd | 18.64 bc | 38.46 d | 52.69 cd | 155.47 l | 2.95 |
| MI-002 | 64.76 g | 18.96 c-e | 17.53 c | 36.49 d | 54.95 c | 178.54 ij | 3.24 |
| MI-004 | 54.40 j | 26.65 a | 18.07 c | 44.72 a | 88.24 a | 171.55 jk | 1.94 |
| MI-008 | 58.07 i | 20.89 bc | 19.18 bc | 40.07 c | 47.70 e-g | 134.32 m | 2.81 |
| MI-009 | 78.62 ab | 11.50 hi | 9.67 fg | 21.17 g | 52.59 cd | 407.05 c | 7.74 |
| MI-016 | 77.88 bc | 12.32 gh | 11.55 d-f | 23.87 fg | 64.70 b | 416.76 b | 6.44 |
| MI-019 | 80.27 a | 9.87 ij | 9.42 g | 19.29 h | 49.89 d-f | 431.76 a | 8.65 |
| MI-020 | 71.75 f | 18.51 de | 8.72 g | 27.23 e | 50.84 de | 186.12 i | 3.66 |
| MI-022 | 72.28 ef | 13.72 g | 17.29 c | 31.01 e | 44.83 g | 235.31 h | 5.24 |
| MI-023 | 57.04 i | 22.46 b | 20.38 ab | 42.84 ab | 40.21 h | 106.59 p | 2.65 |
| MI-024 | 80.53 a | 8.93 j | 8.60 g | 17.53 h | 47.93 e-g | 394.85 d | 8.23 |
| MI-025 | 75.63 cd | 12.64 gh | 10.61 e-g | 23.25 f | 52.40 cd | 315.86 e | 6.02 |
| MI-026 | 74.18 de | 14.26 g | 13.46 d | 27.72 f | 47.33 e-g | 251.12 g | 5.30 |
| MI-028 | 61.27 h | 16.58 f | 18.46 bc | 35.04 d | 34.32 i | 123.64 no | 3.60 |
| MI-030 | 63.18 gh | 17.30 ef | 20.32 ab | 37.62 d | 35.95 i | 125.76 mn | 3.49 |
| MI-043 | 61.27 h | 17.46 cd | 19.35 bc | 36.81 d | 36.11 i | 115.38 op | 3.19 |
| MI-044 | 74.47 de | 11.48 hi | 11.58 d-f | 23.06 fg | 46.66 fg | 319.19 e | 6.84 |
| MI-045 | 75.64 d | 10.71 h-j | 12.32 de | 23.03 g | 47.96 e-g | 305.62 f | 6.37 |
| MI-047 | 64.50 g | 16.40 f | 18.12 c | 34.52 d | 45.60 g | 165.33 k | 3.62 |
| MI-048 | 57.57 i | 20.32 cd | 21.33 a | 41.65 b | 45.34 g | 115.22 op | 2.54 |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
Table 11. Stone characteristics of 20 mango germplasm
| Germplasm | Stone weight (g) | Stone size (cm) | Pulp to stone ratio | ||
| Length | Breadth | Thickness | |||
| MI-001 | 46.41 cd | 8.68 e-g | 3.70 e | 2.61 b-d | 0.29 |
| MI-002 | 45.36 c-f | 8.54 fg | 5.40 cd | 3.11 b-d | 0.25 |
| MI-004 | 56.83 a | 8.46 fg | 6.02 a | 2.88 b-d | 0.33 |
| MI-008 | 43.94 d-h | 8.77 d-g | 5.35 cd | 3.30 a-c | 0.32 |
| MI-009 | 48.30 c | 10.23 b-d | 5.68 bc | 2.97 b-d | 0.12 |
| MI-016 | 57.39 a | 10.80 ab | 5.77 b | 2.79 b-d | 0.13 |
| MI-019 | 47.68 cd | 9.37 c-f | 5.74 b | 1.36 e | 0.11 |
| MI-020 | 21.72 k | 8.77 d-g | 5.57 b-d | 2.50 cd | 0.11 |
| MI-022 | 52.65 b | 11.16 a | 6.11 a | 3.47 ab | 0.22 |
| MI-023 | 41.17 gh | 7.98 g | 4.55 de | 3.04 b-d | 0.38 |
| MI-024 | 47.10 cd | 10.69 ab | 4.54 b-d | 2.35 d | 0.12 |
| MI-025 | 43.86 d-h | 7.81 d-g | 4.44 de | 2.83 b-d | 0.13 |
| MI-026 | 44.29 c-g | 8.61 e-g | 5.13 cd | 4.05 a | 0.17 |
| MI-028 | 40.03 hi | 9.77 c-f | 5.35 cd | 2.90 b-d | 0.32 |
| MI-030 | 37.15 ij | 6.87 g | 4.37 e | 2.26 d | 0.29 |
| MI-043 | 34.60 j | 8.35 fg | 4.49 de | 3.13 b-d | 0.30 |
| MI-044 | 46.05 c-e | 9.34 d-f | 5.44 cd | 3.19 b-d | 0.14 |
| MI-045 | 48.17 c | 9.79 c-e | 4.86 de | 2.50 cd | 0.15 |
| MI-047 | 42.31 e-g | 8.58 e-g | 4.39 e | 2.77 b-d | 0.25 |
| MI-048 | 41.51 f-h | 10.50 bc | 5.16 cd | 3.04 b-d | 0.36 |
Note: Variety means having the same letter are statistically identical and those having different letter are statistically different from each other at P ≤ 0.05
4.3 Isozyme electrophoresis
Electrophoresis means migration of suspended particles such as protein macromolecules under the influence of an electric field i.e. separation of a mixture of electrically charged molecules in an electric field. Protein electrophoresis is the migration of protein under the influence of an electric field. Isozymes are all functionally similar forms of enzymes including all polymers of sub-units produced by different gene loci or by different alleles at the same locus. To detect the gene level variation among the 20 mango accessions peroxidase isozyme analysis was performed.
Zymogram analysis
Zymogram was obtained from Rf values, shown in figure 1 & 2. After the electrophoresis, the gels were stained for peroxidase activities and two or more bands were observed. Based on the Rf values of peroxidase band generated in 20 mango accessions, a zymograms was obtained. The 20 accessions were classified on the basis of their zymogram patterns with the Rf values. There are 10 bands of peroxidase falling into 5 zymotype classes viz. P1, P2, P3, P4 and P5.
Plate 18. Isozyme pattern of peroxidase in mango accession from 11-20
Table 12. Distribution of peroxidase band among different mango zymotype
Zymotypes | Rf values of bands | No. of bands | |||||||||
0.13-0.16 | 0.16-0.20 | 0.20-0.21 | 0.21-0.45 | 0.45-0.46 | 0.46-0.62 | 0.62-0.64 | 0.64-0.67 | 0.67-0.70 | 0.70-1.00 | ||
P1 | √ | – | √ | – | – | – | – | – | – | 2 | |
P2 | √ | √ | – | √ | – | – | – | – | – | – | 3 |
P3 | √ | – | √ | – | √ | √ | – | – | – | – | 4 |
P4 | √ | – | √ | – | – | √ | – | √ | – | √ | 5 |
P5 | √ | – | √ | – | √ | √ | √ | – | √ | – | 6 |
Total | 5 | 1 | 4 | 1 | 2 | 3 | 1 | 1 | 1 | 1 | 20 |
Band frequency (%) | 25 | 5 | 20 | 5 | 10 | 15 | 5 | 5 | 5 | 5 | |
Note. ‘√’ indicates presence and ‘-’ indicates absence of band in respective Rf value.
Band one lies between the Rf values of 0.13 – 0.16, band two lies between the Rf values of 0.16 – 0.20, band three lies between the Rf values of 0.20 – 0.21, band four lies between the Rf values of 0.21 – 0.45, band five lies between the Rf values of 0.45 – 0.46, band six lies between the Rf values of 0.46 – 0.62, band seven lies between the Rf values of 0.62 – 0.64, band eight lies between the Rf values of 0.64 – 0.67, band nine lies between the Rf values of 0.67 – 0.70 and band ten lies between the Rf values of 0.70 – 1.00.
Eiadthong et al. (1998) found polymorphism in different enzyme system (Isocitrate dehydrogenase, IDH and phosphoglucose isomerase, PGI) examined and intracultivar variation of the same cultivar collected at different locations was confirmed in banding patterns.
Table 13. Observed number of electrophoretic zymotypes of peroxidase isozyme.
Zymotypes | Total number of accessions | Percentage (%) |
P1 | 8 | 40 |
P2 | 2 | 10 |
P3 | 4 | 20 |
P4 | 1 | 5 |
P5 | 5 | 25 |
Five electrophoretic zymotypes (P1, P2, P3, P4 and P5) were observed in peroxidase enzyme system formed by 10 bands at different Rf values varying from 0.13 to 0.70 (Table 12). Zymotypes P1 was present in maximum accessions (8) followed by P5, P3 and P2 had 5, 4 and 2 accessions, respectively. On the other hand, P4 showed the lowest appearance having only 1 accession.
Zymotype P1 was the most frequent which included 40% of the total accessions followed by zymotypes P5, P3, P2 and P4 included 25%, 20%, 10% and 5%, respectively.
Table 14. Zymogram patterns of peroxidase isozymes
Zymogram type | Description | Accessions sharing the zymogram |
P1 | Band 1,3 present and |
band 2,4,5,6,7,8,9,10 absentMI-002, MI-008, MI-009, MI-011, MI-012, MI-015, MI-019, MI-020
P2
Band 1,2,4 present and
band 3, 5, 6, 7, 8, 9, 10MI-003, MI-007
P3
Band 1, 3, 5, 6 present and
band 2, 4, 7, 8, 9, 10 absentMI-005, MI-010, MI-014, MI-017
P4
Band 1, 3, 6, 8, 10 present and
band 2, 4, 5, 7, 9 absentMI-001
P5
Band 1, 3, 5, 6, 7, 9 present and
band 2, 4, 8, 10 absentMI-004, MI-006, MI-013, MI-016, MI-018
From distribution of peroxidase bands among the zymotypes of 20 mango accessions, it was observed that bands having Rf values 0.13 – 0.16 and 0.16 – 0.21 were most common for all accessions and frequency both Rf values were 25% and 20%, respectively. The lowest band frequency was 5% with the Rf value 0.70. Thus the accession had wide range of genetic variation. According to Yadav (1997), isozymes analysis of 200 polyembryonic mango varieties revealed that there were 34 allels which showed monomorphism while 19 showed polymorphism.
* H I E R A R C H I C A L C L U S T E R A N A L Y S I S *
Rescaled Distance Cluster Combine
Genotype 0 5 10 15 20 25
number +———+———+———+———+———+
MI-047 òø
MI-048 òú
MI-002 òú
MI-025 òú
MI-030 òú
MI-022 òôòò I òòòòòòòòòòòòòòòòòòòòòòòòòòø
MI-024 òú ó
MI-020 ò÷ ùòòòø
MI-028 òø ó ó
MI-044 òú ó ùòòòòòø
MI-009 òôòò II òòòòòòòòòòòòòòòòòòòòòòòòò÷ ó ó
MI-023 ò÷ ó ó
MI-001 òòòò III òòòòòòòòòòòòòòòòòòòòòòòòòòòò÷ ùòòòòòø
MI-043 òø ó ó
MI-045 òú ó ó
MI-008 òôòò IV òòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòò÷ ó
MI-016 òú ó
MI-026 ò÷ ó
MI-004 òûòò V òòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòòò÷
MI-019 ò÷
Dendrogram analysis:
The dendrogram constructed from Nei’s (1978) genetic distance has been presented in Fig. 3. The dendrogram showed that the 20 accessions were grouped into five clusters namely cluster-I, cluster-II, cluster-III, cluster-IV and cluster-V. Cluster-I consisted of eight accessions (MI-047, MI-048, MI-002, MI-025, MI-030, MI-022, MI-024 and MI-020), cluster-II contained four accessions (MI-028, MI-044, MI-009 and MI-023), cluster-III contained one accession (MI-001), cluster-IV contained five accessions (MI-043, MI-045, MI-008, MI-016 and MI-026) and cluster-V contained two accessions (MI-004 and MI-019).
The accessions collected from same area were distributed in different cluster which indicated that there is no relationship between genetic diversity and geographical distribution of the mango accessions. The accession number which from same group were closely related and there were low genetic variation. On the other hand, between the groups genetic variation was high.
SUMMARY AND CONCLUSION:
The experiment was carried out with 20 mango germplasm involving each of 50 different characters at the Germplasm (GP) Centre of Fruit Tree Improvement Project (FTIP), Department of Horticulture and the Laboratory of Department of Genetics and Plant Breeding, BangladeshAgriculturalUniversity, Mymensingh during March 2004 and August 2005. The objectives of the experiment were to evaluate the morphological and physiological characteristics of mango under the agro-ecological conditions of Mymensingh. The experiment was laid out in Randomized Complete Block Design (RCBD) with three replications.
Data on morphological as well as physiological characters were studied. The results indicated that the flower bud emergence was the earliest (December, 05) in MI-019 and latest in the MI-008 germplasm. Again panicle emergence was first noticed in MI-019 while MI-008 (February, 06). Flower was opened first in MI-019 (January, 18) and MI-008 was the latest (February, 22). Full blooming was first noted in MI-019 (February, 05) and last in MI-004 (March, 17). The germplasm MI-023 and MI-022 were the earliest and latest, respectively in harvesting time. On the other hand, maximum days were required for maturity of MI-019. The shortest period from flowering to harvest was noted in MI-023. Panicle color in most of the germplasm varied from light green to dark red but crimson colored panicle was observed in MI-020 and in general cases panicles were broadly pyramidal in shape. Most of the germplasm had secondary branches in their panicles except MI-016 and MI-045 which had tertiary branches as well. MI-022 produced the maximum number of panicles per shoot, whereas minimum was in MI-043. The longest panicle was noted in MI-045 and the shortest panicle was found in MI-002. The highest number of main branches was in MI-045 and MI-002 had the lowest number of main branches. Maximum percentage of male flower was noted in MI-002 and the lowest male flower was recorded from MI-025. MI-025 had the highest percentage of bisexual flower and minimum was in MI-002. Maximum unopened flower per panicle was in MI-044 and minimum was in MI-002. MI-019 produced the highest number of initial fruits per panicle whereas it was the lowest in MI-008. The highest number of final set of fruit was in MI-028 while MI-030 showed the lowest. In respect of fruit harvest maximum was recorded in MI-047 and the minimum was in MI-044.
Wide variations were observed among the germplasm in terms of qualitative characters namely fruit shape, external appearance, skin color, peeling quality, skin thickness and pulp color. On the basis of these qualitative characters MI-028 was the best one among the germplasms. The heaviest and lightest fruits were recorded in MI-016 and MI-043. Higher edible portion was recorded in MI-024, MI-019 and MI-009 whereas maximum non-edible portion was noticed in MI-004. The germplasm MI-004 possessed the maximum peel per cent while MI-048 contained proportionately the heaviest stone.
In isozyme analysis, leaf sample of the accessions was used, the electrophoresis of the proteins of the leaves was carried out using PAGE technique and the gels were stained with peroxidase isozyme and zymogram analysis was performed. Ten bands were found at different Rf values and band one and two were found in all the accessions.
The result of the present experiment revealed that a wide variability exists among the collected mango accessions. From the result of the experiment the following conclusions can be drawn.
- Wide variability exists among the mango accessions used in this experiment. These variabilities could be used for future breeding programme of mango in our country.
- Fruit and seed characters of mango are positively and significantly correlated with the fruit yield.
- Only the peroxidase isozyme was used for molecular characterization. But for getting more authentic results other isozymes such as Esterase (ET), Glutamate Oxaloacetate Transaminase (GOT), Malate Dehydrogenase (MDH) should be included in future study.
- Recent molecular techniques such as RAPD, RFLP, SSR should be used for proper identification of the germplasm at molecular level.
- Further collection of mango germplasm should be continued for getting more variability in respect of desired traits.