Infusion Unit: Large Volume Parenteral Formulations
Beximco Pharma’s intravenous (IV) fluid manufacturing plant may be regarded as one of the most technologically advanced plants in the world. The plant was designed and installed in collaboration with Pharmaplan of Germany, a sister concern of Fresenius AG of Germany.
In designing the whole process, special care has been taken by providing absolute sterile manufacturing conditions. The prime feature of the process is that there is no human physical contact with the product at any given time. This has been ensured by way of a series of fully automated manufacturing procedures including robotics. The bottle pack aseptic system (Form-Fill-Seal or FFS) is a unique and innovative manufacturing technology. Plastic bottles are blow moulded, filled with the solution and sealed under sterile conditions, in a single working cycle where there is no environmental exposure or human contact during manufacturing. The IV fluids are presented in a scientifically designed bottle where there is an extra protective eurohead cap and a resealable rubber disk. The whole process is performed in a class 100 clean room. The air inside this room is cleaned up to 100 particles per cubic feet passing it through HEPA (High Efficiency Particulate Air) filters.
Thus, an advanced sterile environment is maintained in manufacturing the IV fluids in order to avoid the entry of bacteria, pyrogen and inert particles into products. This ensures the highest standards of quality and purity in order to ensure the highest level of safety.
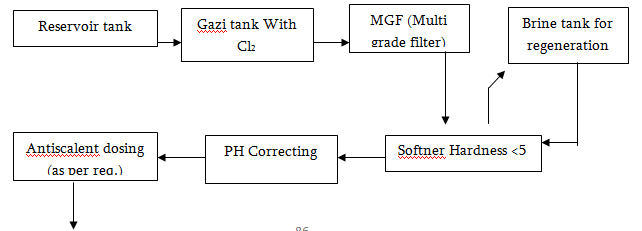
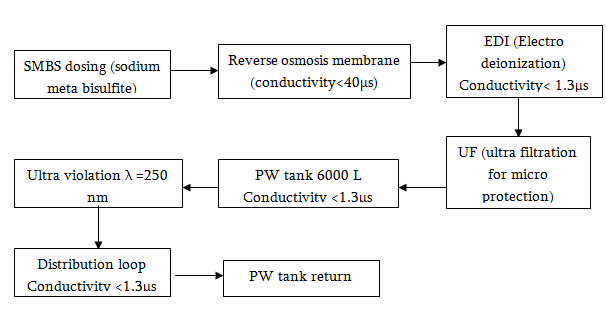
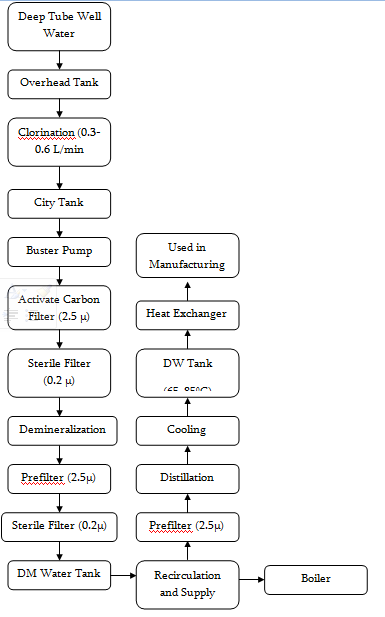
Water Treatment Process
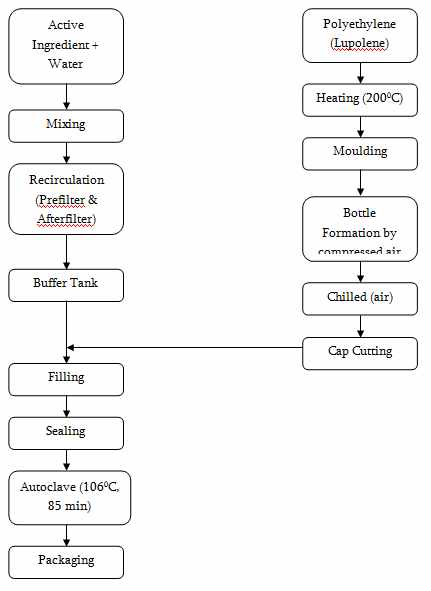
Manufacturing and Packaging Steps
HVAC System
The HVAC system means Heating ventilation and Air conditioning system. The main objective of HVAC system is to maintain a constant temperature and a constant RH in the infusion production area, microbiology laboratory and the office rooms. It is the most modern concept of air conditioning which is the most special features in the infusion unit. HVAC is needed in Pharmaceuticals:
- To maintain specified temperature.
- To maintain specific relative humidity. (Less than 40% for some hygroscopic material like ranitidine).
- To remove dust particle from production area.
- To maintain proper airflow to the rooms ensuring that cross contamination does not occur.
- To prevent microbial contamination in some area by maintaining particle size within the tolerance range. (Using the HEPA Filters etc).
The process is maintained in the different area by controlling the air pressure (positive and negative).
Microbiology Laboratory
According to the ICH guidelines the microbiological test of raw materials and finished product is an important parameter of the quality drug. Accordingly the Infusion unit has a special microbiology laboratory in order to carry out the microbiological tests of several sensitive products.
The microbial test program includes the following:
- Test of raw material and packaging material.
- Test of finished product
- Environmental control
- Purified water test
- Monitoring of surfaces
- Personnel hygiene
The microbiology laboratory of contains the following separate rooms:
¨Testing room & Incubation room:
¨ Media preparation room:
There is also a store room to store the reagents, cultures, standard microbes, chemicals and other testing instruments in a required condition
The total testing procedure can be summarized as follows:
Sample collection
Media preparation
Culture preparation
Incubation
Colony count
Identification
Final result
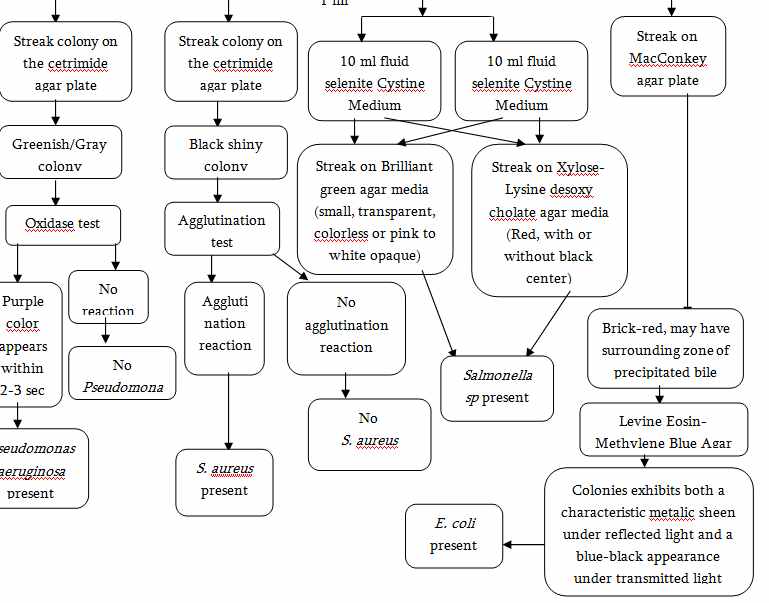
1. Test of Raw and Packaging Materials
Microbial limit test is performed which include:
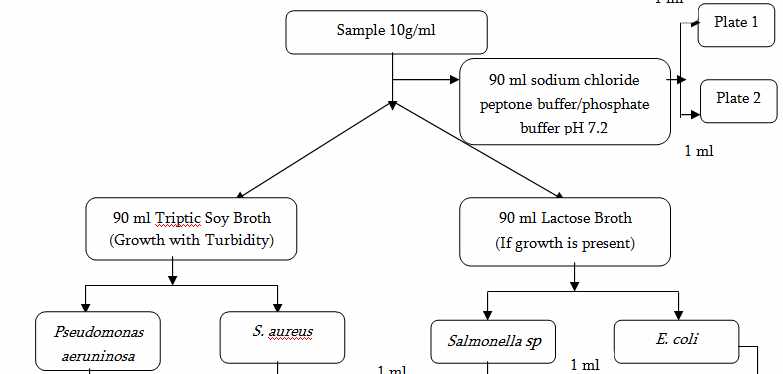
1)Total aerobic viable count
2)Total aerobic bacterial count
3)Total fungal count
4)Presence or absence of Pathogens (E.coli, Salmonella sp. , P. aeruginosa, S. aureus)
Microbial Limit Test for Raw Materials and Finished Products (USP)
2. Test of Products
Bioburden check of intermediate products. It is done with the help of membrane filtration method. First 4 and last 4 bottles are tested for microbes.
Sterility test. 20 bottles of products are collected from critical points of the autoclave and are incubated for 14 days and observed.
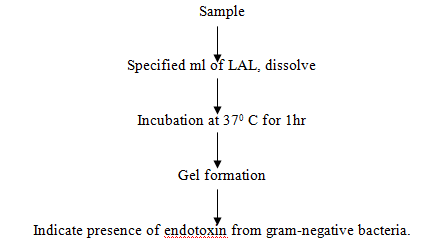
Bacterial endotoxin test/ LAL test. It is an in-vitro test method for pyrogen. It has been developed utilizing the gelling property of the lysate of amoebocytes of limulus polyphemus. The flow diagram of LAL test is given below:
3. For the Environmental inspections following tests are performed
i) Settle Plate Method
ii) Air Sampler
4. Purified Water Test
It is done by membrane filtration method. Water is tested from 12 different points daily and 18 different points weekly.
5. Monitoring of surfaces
It is performed by:
i)Contact Plate Method
ii)Swap Test
6. Monitoring of personnel hygiene
Hands, gloves, appearances/clothes are tested by contact plate method.
List of Instrument of Infusion Unit
Sl. | Name of Instrument | Manufacturer/ Supplier | Origin | Uses |
1 | Deep Tube-well Aeration Chamber Sand Bed Filter | Sigma Eng. | Bangladesh | Raw water, supplied water for DM plant |
2 | Raw water reservoir (Overhead tank – 5000L) | Beximco Infusions Ltd. | ||
3 | Dosage station for chlorine with pump and container | LMI MILTON ROY | USA | Killing microorganisms |
4 | Filter housing for raw water | Pall | ||
5 | City water tank (2000L) | Beximco Infusions Ltd. | ||
6 | Booster pumps (3000L/H) | Grundfos | Germany | |
7 | Activated carbon filter (1100L) | Hager + Elsasser, GmbH | Germany | Replace organic substance from water and declorinate water for DM plant area |
8 | Filter house (2.5μ) | Pall | ||
9 | Filter house (0.22μ) | Pall | ||
10 | Demineralization plants (1 and 2) (Anion and cation exchanger) | Hager + Elsasser, GmbH | Germany | Production of DM water for distillation plants, boiler etc. |
11 | Mixed bed filter (3.5m3/h) | Hager + Elsasser, GmbH | Germany | Polishing DM waters |
12 | Filter housing (0.22μ) | Pall | ||
13 | Filter housing (0.22μ) | Pall | ||
14 | Regeneration and neutralization system (9000L) | Pharma Plan | Germany | |
15 | DM water tank (4870L) | Pharma Plan | Germany | |
16 | Distillation Plant 01 | EKSTROM & SONS | Sweden | |
17 | Distillation Plant 02 | KEMITERM ENGNEERING A/S | Sweden | Production of WFI and pure steam |
18 | Distillate Storage Tank 01 (7000 L) | Pharma Plan | Germany | |
19 | Distillate Storage Tank 02 (7500 L) | Getinge | Sweden | |
20 | Steam Boiler (2.6 T/H) | StandardKessel | Germany | Provide Technical steam |
21 | Oil Free Air Compressor 01 & 02 | Mehrer | Operating electronumeric valves & preparing sterile compressed air | |
22 | Water Chiller 01 | Finetee Century | Korea | Supply chilled water for HVAC & Production |
23 | Water Chiller 02 | Linde Aktiengesellschaft | Germany | Do |
24 | Water Chiller 03 | Kyungwon-Century Co. Ltd. | South Korea | Do |
25 | Air Handling Unit 01 & 02 | MEISSNER & WURST | Germany | Supply & maintain clean class, positive air pressure |
26 | Cooling Tower 01 | Linde AG, Mainz | Germany | Provide cooling air |
27 | Cooling Tower 02 | BSC | Singapore | Provide cooling air |
28 | Injection Moulding Machine | PO Yuen (To’s) Machine Factory | Hong Kong | Produce Cap |
29 | Preparation Tank 01 & 02 | Pharma Plan | Germany | |
30 | Preparation Tank 01 & 02 | Getinge | Sweden | |
31 | Fully Automatic Bottlepack Machine for 1000ml & 100ml Bottles | Rommelag | Germany | Bottlepacking |
32 | Cap Welding Machine for 1000ml & 100ml Bottles | Pharma Plan | Germany | Cap welding |
33 | Fully Automatic Bottlepack Machine for 500ml & 250ml Bottles | Rommelag | Germany | Bottlepacking |
34 | Cap Welding Machine for 500ml & 250ml Bottles | Pharma Plan | Germany | Cap Welding |
35 | Spray Type Autoclave | Sauter-Sulgen | Germany | Autoclaving |
36 | Spray Type Autoclave | Getinge | Sweden | Autoclaving |
37 | Pressing Conveyor Belt | Pharma Plan | Germany | Leak testing |
38 | Conveyor Belt | Pharma Plan | Germany | Conveying |
39 | Visual checking Devices | |||
40 | Conveyor Belt | Pharma Plan | Germany | Conveying |
41 | Labeling Machine with Batch No. Printer | Pharma Plan | Germany | Labeling |
42 | Ink Jet Printer | Image | France | Printing on Overpack |
Engineering Department
Major functions
The main aim of this dept is to support the production department for smooth running. Machineries of this department are of 2 types-
- Production machinery
- Utilities
A. Operation & maintenance of utility machineries are as follows:
a) Power supply
b) water system
c) Steam
d) Central compressor
e) Central air conditioning system
f) Dehumidification
g) Fork lift
h) Hand pallet truck
i) Vacuum cleaner
j) Gas
B. Maintenance of production machinery.
a) Scheduled maintenance
b) Troubleshooting or breakdown maintenance
C. Maintenance of Q.C machinery.
Utility Machineries
Power station
Machines | Name | Origin | Capacity |
| Generator-1 | WAUKESHA | U.S.A | 920 KW |
| Generator-2 | CATERPILLAR | U.S.A | 1020 KW |
| Generator-3 | WAUKESHA | U.S.A | 900 KW |
| Generator-4 | WAUKESHA | U.S.A | 900 KW |
| Diesel generator | DETROIT DIESEL ALLISON |
Primary Requirements to run through Gas engine in the power station:
- Gas
- DM Water
- Soft Water
- Lube Oil
- Battery
- Compressed Air
- Air
Boiler
There are 2 boiler machines.
| Machines | Name | Origin | Capacity |
| Boiler-1 | Cleeve Brooks | U.S.A. | 3 Ton steam/hr |
| Boiler-2 | Cleeve Brooks | U.S.A. | 2 Ton steam/hr |
Hapa Foil Printer
Model: Hapa 226
Origin: Switzerlan
Water Purifying System