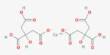
Rare earth elements (REEs) can be difficult to recover from environmental water because they are typically present in low concentrations and are frequently found in complex mixtures with other elements and compounds.
Using baker’s yeast with a phosphate group added, a research team was able to selectively recover trace rare earth elements in synthetic seawater and environmental water, such as hot spring water. The phosphorylated yeast is expected to be used as a material for recovering useful metals and removing toxic metals, helping to realize a metal resource-circulating society.
The demand for precious metals and rare earths is expected to rise in the coming years. To ensure a consistent supply, recycling from precision equipment and recovering from seawater and hot spring water are required due to limited production areas.
Through environmental purification, this new technology is expected to contribute to the realization of a metal resource-circulating society and a safe society. In the future, we will continue to conduct experiments on a variety of environmental water with the goal of developing a system capable of treating large quantities of metal resources in continuous operation.
Professor Masayuki Azuma
A research group led by Professor Masayuki Azuma and Associate Professor Yoshihiro Ojima of the Osaka Metropolitan University Graduate School of Engineering has successfully developed an adsorbent material that can selectively recover rare earth elements (REEs) using environmentally friendly and inexpensive baker’s yeast and trimetaphosphate, which is used as a food additive.
One common method for recovering REEs from environmental water is through a process called ion exchange. This involves passing the water through a resin that contains functional groups that can selectively bind with the REEs. Once the REEs have been adsorbed onto the resin, they can be eluted using an appropriate chemical solution.
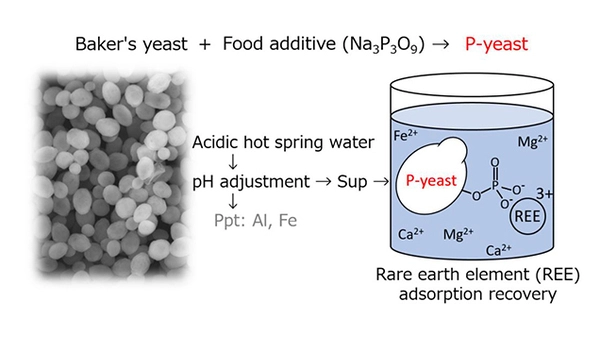
The research group conducted experiments using synthetic seawater and hot spring water to evaluate the performance of this material in a real environment. As a result, it was confirmed that the material can selectively adsorb REEs even when using hot spring water with an REE concentration of several to several tens of ppb (?g/L) and a very high content of other components.
Overall, recovering REEs from environmental water can be a complex process, and the specific method used will depend on a variety of factors, including the concentration of the REEs in the water, the presence of other contaminants, and the desired purity of the final product.
“Through environmental purification, this new technology is expected to contribute to the realization of a metal resource-circulating society and a safe society. In the future, we will continue to conduct experiments on a variety of environmental water with the goal of developing a system capable of treating large quantities of metal resources in continuous operation,” Professor Azuma said.