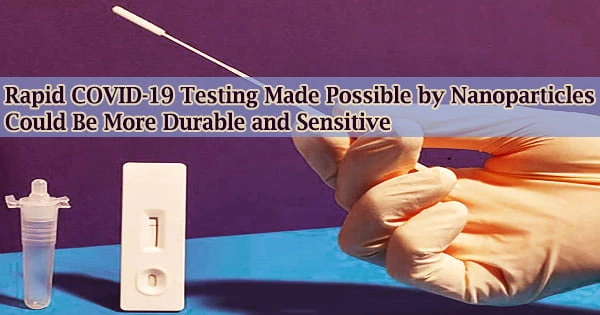
One can easily and quickly find out if they have COVID-19 with rapid antigen tests. However, due to their low sensitivity, antibody-based diagnostics may miss early infections with low viral loads.
Researchers have now created a quick test for SARS-CoV-2 detection using molecularly imprinted polymer nanoparticles rather than antibodies, as reported in ACS Sensors. Compared to antibody-based testing, the new test is more sensitive and reliable in challenging environments.
The reverse transcription-polymerase chain reaction remains the gold standard test for COVID-19 diagnosis (RT-PCR). Although this test is extremely sensitive and specific, it also typically takes 1-2 days to provide a result, costs a lot of money, and calls for specialized lab equipment and qualified staff.
Rapid antigen tests, on the other hand, can be administered at home and require only 15 to 30 minutes. They are insensitive, nevertheless, which occasionally yields false negatives.
Additionally, the tests rely on antibodies against SARS-CoV-2 for detection, which are sensitive to changes in pH and temperature. Molecularly imprinted polymer nanoparticles (nanoMIPs), rather than antibodies, are used in the COVID-19 test developed by Marloes Peeters and Jake McClements of Newcastle University, Francesco Canfarotta of MIP Diagnostics, and associates.
By leaving molecular impressions, sometimes known as molds, in the nanoparticles, the researchers were able to construct nanoMIPs that were specific for a short peptide or portion of the SARS-CoV-2 spike protein. The imprinted peptide and, thus, the complete protein may be recognized and bound by these nanoscale binding cavities because of their size and structure.
They adhered to printed electrodes the nanoparticles that bonded to the peptide most firmly. After demonstrating that the nanoMIPs could bind SARS-CoV-2, the researchers created a 3D-printed prototype device that can detect binding by monitoring temperature fluctuations.
The liquid ran over the electrode as the team added samples from seven patient nasopharyngeal swabs, and they saw a shift in temperature for the samples that had previously tested positive for COVID-19 by RT-PCR.
The test took only 15 minutes, and preliminary findings showed that it was 6,000 times more sensitive than a commercial quick antigen test for detecting SARS-CoV-2. The test may last longer in hot regions thanks to the nanoMIPs’ resistance to warm temperatures and acidic pH, which may make it effective for monitoring SARS-CoV-2 in wastewater and saliva samples.
The test needs to be tested on many more patient samples, according to the researchers, in order to demonstrate that it has a lower false negative rate than current fast antigen tests.
The authors acknowledge funding and support from Newcastle University, the Rosetrees Trust, the Wellcome Trust, MIP Diagnostics and the Fonds de la Recherche Scientifique.