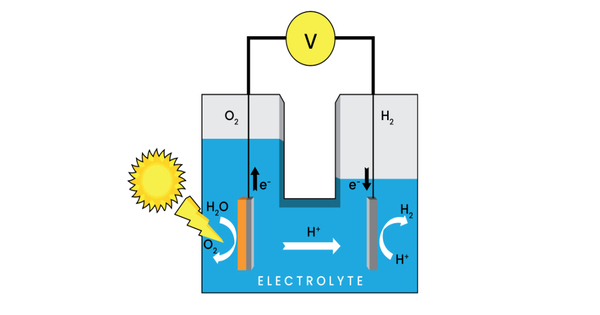
The term “photoelectrochemical cell” refers to one of two types of devices. A photoelectrochemical system, also known as a photoelectrochemical device, is a type of device that uses photoelectrochemistry to convert solar energy into chemical energy or electricity. It’s a hybrid system that combines elements of solar cells and electrochemical cells, with light driving electrochemical reactions.
The first generates electrical energy in the same way that a dye-sensitized photovoltaic cell does, and thus meets the standard definition of a photovoltaic cell. The second type is a photoelectrolytic cell, which is a device that uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, such as the electrolysis of water to produce hydrogen.
Both types of devices are solar cells in the sense that a photoelectrochemical cell uses the photoelectric effect (or, more precisely, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power or into something that can be easily used to produce electrical power (hydrogen, for example, can be burned to produce electrical power).
Here’s how a basic photoelectrochemical cell works:
- Photoabsorption: The cell consists of a semiconductor material, often a metal oxide or a compound semiconductor, that can absorb photons (light particles) and generate electron-hole pairs when exposed to sunlight. The semiconductor’s bandgap determines the range of light wavelengths it can absorb.
- Charge Separation: When light is absorbed by the semiconductor, electron-hole pairs are generated. The electrons move to the conduction band while the holes move to the valence band.
- Electrochemical Reactions: The electrons produced can be harvested and used in electrochemical reactions at one electrode (the cathode), while the holes produced can be used in reactions at the other electrode (the anode). This is accomplished by employing appropriate redox reactions or other chemical reactions.
- Electricity Generation or Chemical Transformation: The generated electrons and holes can be used to generate an electric current (for electricity generation) or to drive chemical reactions that produce useful products (such as the generation of hydrogen from water splitting) depending on the design and purpose of the PEC cell.
PEC cells could be used in renewable energy conversion, energy storage, and environmental remediation. They can be used to split water to produce hydrogen fuel, to reduce carbon dioxide to produce fuels and chemicals, and even to create self-sustaining systems that can purify water while producing energy.
However, finding suitable semiconductor materials with efficient light absorption, charge separation, and catalytic properties for the desired reactions is one of the challenges associated with PEC cells. Furthermore, managing corrosion and material stability in harsh electrochemical environments is a significant challenge.