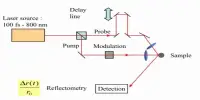
Phosphorescence, like fluorescence, is a type of photoluminescence. When exposed to shorter wavelength light (radiation), a phosphorescent substance will glow, absorbing the light and reemitting it at a longer wavelength. A phosphorescent material, unlike fluorescence, does not immediately reemit the radiation it absorbs. A phosphorescent material, on the other hand, absorbs some of the radiation energy and reemits it for a much longer period of time after the radiation source is removed.
Phosphorescence is a phenomenon in which a substance absorbs energy and then emits light for an extended period of time after the energy source is removed. It is a type of photoluminescence, which is the emission of light from a material after it has absorbed photons.
In general, there is no clear distinction between the emission times of fluorescence and phosphorescence (i.e., if a substance glows under a black light, it is considered fluorescent; if it glows in the dark, it is frequently referred to as phosphorescent).
The absorption of energy raises electrons in the material to higher energy levels in phosphorescence. These electrons then undergo a transition back to their original energy levels, releasing energy in the form of light. However, unlike fluorescence, which emits light immediately, phosphorescence involves a delay between absorption and emission. Depending on the material, the delay can range from a fraction of a second to hours or even days.
In a modern, scientific sense, the phenomena are typically classified by the three different mechanisms that produce the light, as well as the typical timescales during which those mechanisms emit light. Whereas fluorescent materials cease to emit light within nanoseconds (billionths of a second) after the excitation radiation is removed, phosphorescent materials may continue to emit an afterglow for several microseconds to many hours.
Phosphorescent materials are typically made up of phosphor-containing substances. These phosphors can be organic or inorganic compounds, and they are frequently doped with impurities to improve their phosphorescence. Zinc sulfide, strontium aluminate, and certain types of phosphorescent pigments used in glow-in-the-dark products are examples of well-known phosphorescent materials.
Phosphorescence can occur naturally in minerals or living organisms, but it can also be artificially induced in a variety of applications. Phosphorescent materials, for example, are used in safety signs, emergency exit signs, and glow-in-the-dark toys. They are also found in some lighting, such as “glow-in-the-dark” paints or coatings. Phosphorescent materials can also be used in scientific research, such as fluorescence microscopy or as markers in biological studies.