According to HBO’s popular zombie TV series “The Last of Us,” in which a fungal mutation disseminated across the world’s food supply causes society to collapse, fungus is currently getting a rather terrible name in popular culture. But, particularly as it relates to the world’s food supply, its crucial role in maintaining the planet’s ecosystems is widely acknowledged.
In order to keep food stocks secure, the agribusiness sector has long worked to improve pathogen-destroying techniques. Fungal may both protect and harm grains and plants.
That’s the focus of a new paper published in Microsystems & Nanoengineering by researchers at the Shih Microfluidics Lab. Scientists have created a brand-new technique for extracting enzymes that break down the cell walls of dangerous microorganisms like nematodes, other fungal infections, or other pathogens that might destroy crops.
In effect, it acts as a biological fungicide and pesticide. The researchers also built a device that makes the technique portable, affordable and easy to use.
“While recent decades have seen improvements in growing strains of mammalian or bacterial cell culture, the techniques used are difficult to apply to fungus due to their specific morphology,” says Kenza Samlali, the paper’s primary author. “So there is a lot of interest in automated systems and microfluidics that can screen for micro-organisms.”
Ph.D. student Chiara Leal Alves co-authored the paper with University of Toronto master’s student Mara Jezernik and Steve Shih, associate professor of electrical and computer engineering.
Studying fungal-based organisms is so hard. The microfluidic method that Kenza and Chiara have designed is truly a breakthrough in the field, especially for determining fungal antagonists.
Shih Microfluidics Lab. Researcher
A difficult organism to keep small
To find a technique to get Clonostachys rosea, a fungus harmful to other fungi, bacteria, and nematodes, to create more crop-protecting catalytic enzymes, the researchers researched it.
Scientists were particularly interested in finding a technique to shield crops from fusarium, a potentially cancer-causing fungus that infects fruits like bananas, squash, and pumpkins as well as grain crops like wheat, rye, and barley.
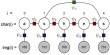
The researchers point out that it is challenging to examine fungi due to their unique characteristics. Fungi have spindly hyphae that can make them challenging to analyze in the kinds of nanoliter droplets utilized in microfluidics since they grow quickly. The researchers created a new substrate to grow the c. rosea on within a droplet.
In a solid-state fermentation technique, this enabled them to study a single spore using the thousands of different types of enzymes that it expressed. They also created a tool that could delicately separate those fungi that produced large amounts of important enzymes while still maintaining the droplets.
Out of the tens of thousands of expressed enzymes, they could then pick out a few dozen individual strains they thought would be most helpful. Scientists were searching for strains that produced the enzymes necessary to break down the cell walls of pathogenic fungus, which are a vital organic resource for a wide variety of crops.
“Studying fungal-based organisms is so hard,” Shih says. “The microfluidic method that Kenza and Chiara have designed is truly a breakthrough in the field, especially for determining fungal antagonists.”
“With a way to culture these in single-cell format, we can now use this technology to screen through thousands of fungal antagonists, which can then be used as biocontrol agents to prevent plant pathogens and diseases. We see immediate use of this in the field of agriculture, but we also see future uses in examining fungal diseases related to human health.”