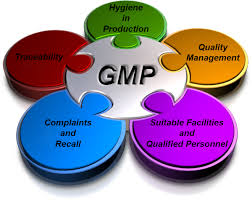
Good manufacturing practice are the practices required in order to conform to tips recommended by agencies that control agreement and licensing pertaining to manufacture and sale made of food, pill products, and active pharmaceutical products. These guidelines produce minimum requirements which a pharmaceutical or the food product supplier must meet to reassure that the goods are of high quality , nor pose any risk towards consumer or general public. A good manufacturing practice is often a system for ensuring that products are continually produced and controlled in accordance with quality standards.
Manufacturing Practice