Clean energy technologies like CO2 electrolyzers, water electrolyzers, fuel cells, redox flow batteries, and ion-capture electrodialysis all depend on ion-transport membranes. These membranes must effectively conduct certain ions while filtering out particular molecules to avoid crossover.
Ion-transport membranes are mostly used in practical modules due to the inexpensive cost, scalability of manufacture, and compact footprint of polymer materials.
However, the existing polymer membranes suffer from a ubiquitous “conductivity-selectivity” trade-off: highly conductive membranes tend to exhibit low selectivity and vice versa. The development of membrane materials that meet the necessary performance requirements is made difficult by this trade-off.
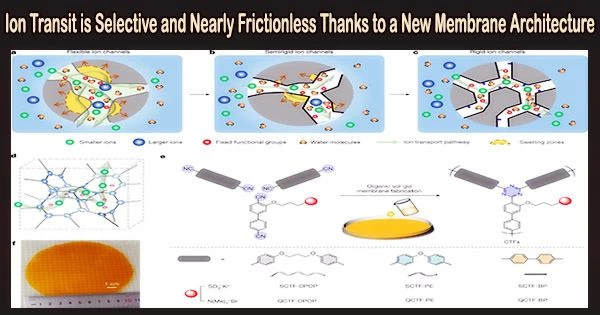
In a study published in Nature on April 26, the research team led by Professor Xu Tongwen and Professor Yang Zhengjin from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS), and their collaborators, proposed a new type of ion exchange membrane triazine framework polymer membranes which can break the conductivity-selectivity trade-off.
The triazine framework polymer membranes shown a significantly improved capability in both anti-swelling and anti-aging, with an incredibly low swelling ratio on water absorption, as compared to traditional materials. They have extraordinarily poor permeability for active materials because of the stiff channels’ great selectivity from size-sieving.
The researchers found that the all-stiff triazine framework polymer membrane (SCTF-BP) had nearly frictionless ion flow and an ion diffusion coefficient that was close to that of water when rigid pore channels’ chemistry was properly controlled. This is made possible by the multi-interaction between ions and membrane and the firm confinement of micropores within stiff pore channels.
As ion-conducting membranes in 2,6-dihydroxy anthraquinone/K4(Fe(CN)6) aqueous organic redox flow batteries, these framework membranes demonstrated both extremely low permeability of active materials and ultrahigh ion diffusivity.
The membrane delivered a neat area-specific resistance as low as 0.17 Ω cm2, and thus enabled stable cell operation at extreme current densities, from 200 to 500 mA cm-2, with both high energy efficiency and high-capacity utilization.
These energy efficiency and capacity utilization results are significantly better than those for otherwise comparable cells built with commercial membranes and cutting-edge ion-sieving membranes. This research emphasizes the value of secondary interactions in creating effective ion-transport membranes.
The suggested design strategy is thought to be broadly applicable since it takes into account a variety of organic reactions and functional monomers that may be used to build polymer frameworks, and it guides the development of membranes that are suitable for their intended use in accordance with the requirements of real-world applications.