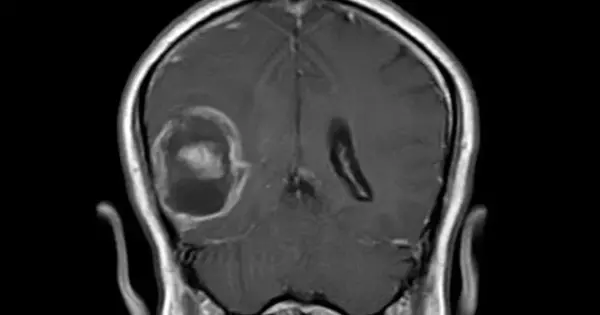
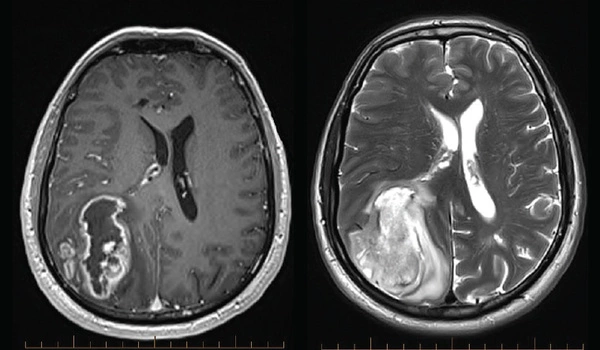
Glioblastoma is a highly malignant brain tumor that begins and grows in the brain. It is a type of glioma tumor, specifically an astrocytoma. Astrocytomas are graded on a scale of one to four, with four being the most aggressive tumor. Glioblastoma is another name for this type of cancer.
In the difficult battle against glioblastoma, scientists are taking cues from viruses on how to make the aggressive cancer more treatable. Their target is SAMHD1, a protein that can protect us from viral infections by destroying an essential building block of DNA required by viruses and cancer to replicate.
However, they discovered that SAMHD1 also has the seemingly contradictory ability of assisting in the repair of double-strand breaks in DNA, which if left unrepaired can be lethal to any cell, including cancer cells, and if mended incorrectly can result in genetic mutations that cause cancer.
“When the DNA breaks, that is what actually interrupts the DNA replication and also the synthesis of proteins, so a double-strand break is lethal for cells,” says Waaqo Daddacha, Ph.D., a cancer biologist at the Medical College of Georgia’s Department of Biochemistry and Molecular Biology.
Cancer cells, which are reproducing much more rapidly than most normal cells, are replicating even faster, so are impacted even more by these DNA breaks, which is why fundamental therapies like radiation and some chemotherapy drugs used to treat cancers make these lethal breaks.
However, the aggressive brain cancer quickly becomes treatment-resistant and the average survival remains at about 15 months, Daddacha says.
When the DNA breaks, that is what actually interrupts the DNA replication and also the synthesis of proteins, so a double-strand break is lethal for cells. Theoretically, since cancer cells divide rapidly and need more dNTP to do that, it seems logical that they would need less of this protein.
Waaqo Daddacha
Daddacha and colleagues now report in the journal Cancers their surprising discovery that in glioblastoma in humans, both SAMHD1 and the essential DNA building block dNTP, which it can destroy, are highly expressed, indicating SAMHD1’s likely importance in the aggressiveness of the brain tumor and raising questions about what it’s doing there.
They were expecting high levels of dNTP because cancers require a steady supply of this building block to maintain their rapid replication and spread, according to Daddacha. They expected low levels of SAMHD1 to be present because dNTP levels were high, and that increasing its levels would help protect against glioblastoma.
They would find the opposite to be true, indicating that like with so many innate properties cancer usurps, glioblastoma likely alters the function of SAMHD1.
“Theoretically, since cancer cells divide rapidly and need more dNTP to do that, it seems logical that they would need less of this protein,” Daddacha says. It also seems logical that SAMHD1 would be protective against cancer, like it is against viruses, Daddacha says.
Based on what they found, the scientists instead decided to reduce SAMHD1 levels and that’s where viruses’ skill at eliminating the multitasking protein came in.
Viruses deploy viral protein X, or Vpx, to literally chop up SAMHD1 so they will have a ready supply of dNTP, a virus skill set first identified in HIV. So, the scientific team used a virus-like particle, called a vector, to deliver Vpx directly to the glioblastoma. These types of viral vectors already are used in people to deliver a variety of therapies, including some of the COVID-19 vaccines.
They discovered that by decreasing SAMHD1, Vpx sensitized brain tumor cells to the chemotherapy drug veliparib, which helps cancer cells block DNA damage repair, and slowed cell growth of this fast-growing brain tumor. It also made the tumor cells more sensitive to temozolomide, or TMZ, another chemotherapy drug commonly used for glioblastoma that disrupts the DNA structure of the cell with the goal of killing it. By lowering SAMHD1, Vpx appeared to lower the innate skill known as homologous recombination, which SAMHD1 promotes and which allows for sound repair of double-strand breaks while also avoiding cell mutations that could lead to cancer.
Reducing SAMHD1 levels enabled the combination therapies, including radiation, used for glioblastoma to work more synergistically as intended, and reconfirmed SAMHD1’s role in enabling glioblastoma’s usual treatment resistance, the scientists write.
In a mouse model with human brain tumor cells, they found that reducing SAMHD1 slowed tumor growth and genetically knocking out SAMHD1 improved survival, the scientific team reports.
As another piece of the puzzle, when the scientists removed SAMHD1, dNTP levels increased slightly but not dramatically, as they had seen in other cell types. This is more evidence that, while SAMHD1 still has some impact on degrading this DNA building block, it clearly has another function in this scenario, according to Daddacha.
He suspects that the true benefit of high SAMHD1 levels in glioblastoma is “self-protection,” which relies primarily on the protein’s innate ability to help repair double-strand DNA breaks. According to Daddacha, there is overwhelming evidence that glioblastoma’s ability to repair double-strand DNA breaks is critical to its treatment resistance, making targeting the repair process logical.
One of the ways cancer takes over a protein for its own purposes is by modifying its function so, for example, it could shift SAMHD1 into primarily DNA repair mode and reduce its natural ability to degrade dNTP, and Daddacha suspects glioblastoma is changing SAMHD1’s function.
“Clearly it’s using it to survive,” Daddacha says, which is likely at least a part of how glioblastoma is so tenacious, and a piece of the big puzzle needed to one day better treat the deadly cancer.
Daddacha’s findings suggest that SAMHD1 can be targeted and eliminated in glioblastoma using the viral protein Vpx, but he cautions that much work remains to be done before the findings and tool can be used to improve glioblastoma treatment. The next step is to learn more about what SAMHD1 does in glioblastoma and how it can coexist with high levels of dNTP, which it should destroy.
“We’ll try to figure out if SAMHD1 is actually degrading dNTP in cancer cells,” Daddacha says. According to him, high levels of SAMHD1 may also help keep high levels of dNTP in check, because everything requires balance.
They are also refining the safe, specific technique of delivering Vpx in the hopes that it will one day be used in humans, as well as determining whether the technique inhibits glioblastoma growth in other ways.
Glioblastoma is a lethal cancer that is always diagnosed as an aggressive, stage 4 tumor, with patients living an average of 15 months after diagnosis, according to Daddacha. The tumor has already progressed by the time symptoms such as headaches and seizures appear.
Surgery to remove as much of the tumor as possible, radiation, and chemotherapy are all standard treatments.