Obesity is a global health issue that puts people at risk for diseases like type 2 diabetes, cardiovascular disease, fatty liver, and even cancer. As a result, it poses a significant hazard to human health and places a significant load on public health systems. Due to the disappointing results of food and physical activity programs, the use of weight reduction medicines has been advocated as a more effective option for long-term obesity management.
According to a new study published in the open-access journal PLOS Biology by Jiang Wei Wu and colleagues from Northwest A&F University in Shaanxi, China, an anti-tumor medicine induces weight reduction in mice at low dosages by activating a natural hunger-suppressing system. The findings provide up a viable new option for the development of anti-obesity medicines.
Growth differentiation factor 15 (GDF15) is a hormone that circulates in response to a number of stimuli, including stress. Previous research has demonstrated that increasing GDF15 causes weight loss, but decreasing it causes obesity.
Increased circulating levels of growth differentiation factor 15 (GDF15) have been shown in rodents and nonhuman primates to reduce food intake and body weight via activation of the hindbrain receptor glial-derived neurotrophic factor (GDNF) receptor alpha-like (GFRAL), suggesting that endogenous induction of this peptide holds promise for obesity treatment.
We believe our findings persuasively imply that camptothecin may have therapeutic effects for obesity and related metabolic diseases. Further research is required to assess its efficacy and safety in advanced models in order to increase the translational impact.
Jiang Wei Wu
The authors used the “Connectivity Map,” a database of gene expression profiles of human cells in response to drug exposure, to look for medicines that could stimulate GDF15 synthesis. They discovered that cells treated to the chemical camptothecin boosted the expression of GDF15. Camptothecin is a recognized inhibitor of a DNA repair enzyme produced from the Asian tree Camptotheca acuminata (hence its use as an anti-tumor drug).
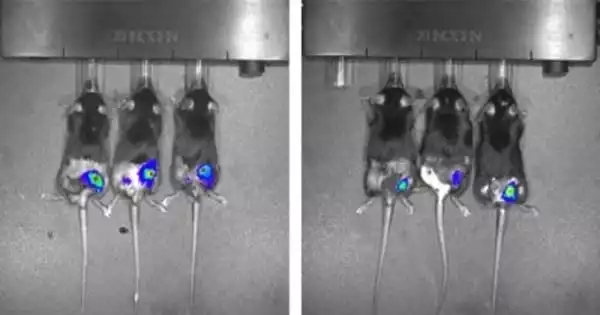
The scientists demonstrated that oral treatment of camptothecin rapidly increased the level of GDF15 in the blood and, over the course of 30 days, lowered food intake by about 12% and body weight by about 11% in obese mice. Camptothecin, on the other hand, did not increase GDF15 in lean mice and had no influence on food intake or body weight.
Camptothecin’s action was specific to GDF15, and GDF15 exerted its effect via its receptor, called GFRAL, the scientists demonstrated, because an antibody against GDF15, as well as cutting down GFRAL expression, prevented weight loss.
Camptothecin was examined in anti-cancer trials but was eventually withdrawn due to safety concerns. Wu stated that its safety as an anti-obesity medicine has yet to be determined, but that the dose used in this study, if scaled up to a human, would be roughly one-thirtieth of the lowest amount used in human anti-cancer trials. Furthermore, the anti-obesity mechanism appears to be distinct from the anti-cancer mechanism, which requires inhibiting the action of the DNA-repair enzyme topoisomerase and operates at a considerably lower drug concentration.
“We believe our findings persuasively imply that camptothecin may have therapeutic effects for obesity and related metabolic diseases,” Wu says. “Further research is required to assess its efficacy and safety in advanced models in order to increase the translational impact.”
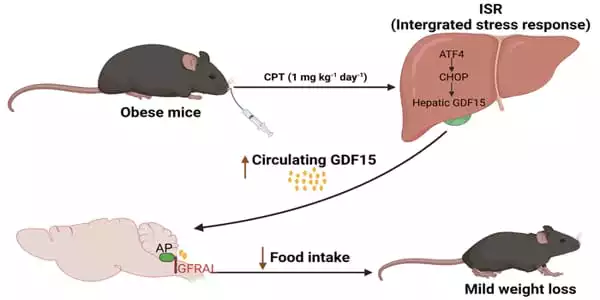
Wu continues, “In this study, we revealed that Camptothecin (CPT), a previously recognized anti-tumor medication by the US National Cancer Institute, is a GDF15 inducer utilizing an in silico drug-screening approach. CPT increases circulating GDF15 via the hepatic ISR route, which activates the GDF15 receptor GFRAL in the hindbrain AP, suppressing food intake and lowering body weight in obese rats.”
GDF15, also known as macrophage inhibitory cytokine-1, has emerged as a novel anti-obesity target. It is a stress-responsive cytokine that is expressed in a range of tissues and secreted into the bloodstream in response to a variety of stimuli as part of a variety of disease processes. In mice and nonhuman primates, increasing GDF15 levels by transgenic overexpression or pharmaceutical treatment result in a significant decrease in body weight. On a high-fat diet (HFD), mice lacking GDF15 grow more obese than wild-type (WT) counterparts. With the recent discovery of GDF15’s particular receptor, glial-derived neurotrophic factor (GDNF) receptor alpha-like (GFRAL), interest in understanding this hormone’s control and researching its therapeutic application in the treatment of obesity has increased.