Genetic variations can affect many elements of human physiology, including the shape and structure of the skull base. The skull base, as a complex structure, is vulnerable to genetic effects during development.
Researchers discovered that a mutation in the gene TBX1 is critical in the formation of the distinctive morphology near the base of the skull. Humans have higher amounts of TBX1 compared to closely related hominins. Low TBX1 levels are also seen in certain hereditary disorders, resulting in altered skull base shape. This work advances our understanding of human disease and evolution.
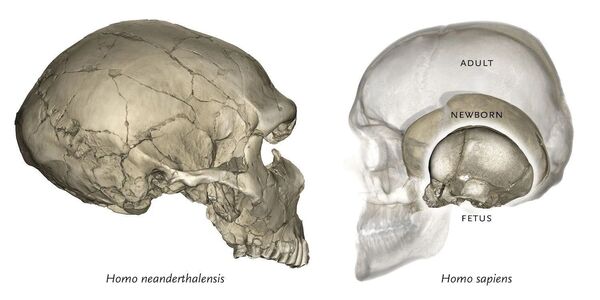
Humans, Homo sapiens, exhibit distinct characteristics when compared to other closely related hominin species and primates, such as the form of the base of the skull. The evolutionary modifications underlying these characteristics played a crucial role in the emergence of our larger brain size. In a recent study published in the American Journal of Human Genetics, researchers from Tokyo Medical and Dental University (TMDU), the University of Helsinki, and the University of Barcelona they investigated a genetic mutation that is responsible for this distinct human skull base morphology.
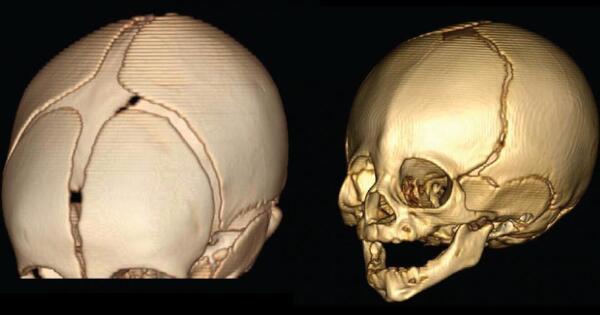
We then employed a mouse model with lower TBX1 expression, which resulted in distinct alterations to the morphology at the base of the skull and premature hardening of a cartilage joint where the bones fuse together, restricting the growth ability of the skull.
Noriko Funato
The majority of chromosomal alterations that happened during human evolution occurred in regions responsible for controlling and regulating gene expression, rather than directly in genes themselves. Variants in these same areas are frequently implicated in genetic disorders, resulting in abnormal gene expression throughout development. Identifying and characterizing such genetic alterations is thus critical to understanding human development and illness.
The evolution of Homo sapiens was aided by the development of the basicranial area, which connects the base of the skull to the bones of the neck, since we acquired a highly flexible skull base that allowed for our larger brain size. As a result, mutations influencing the development of this region are believed to have played a substantial role in our evolution.
First, the team searched for variants in just a single letter of the DNA code, called single nucleotide polymorphisms (SNPs), that caused different regulation of genes in the basicranial region in Homo sapiens compared with other extinct hominins. One of these SNPs stood out, located in a gene called TBX1.
They then used cell lines to show that the SNP, called “rs41298798,” is located in a region that regulates the expression levels of the TBX1 gene, and that the “ancestral” form of the SNP, found in extinct hominins, is associated with lower TBX1 expression, while the form found in Homo sapiens gives us higher levels of TBX1.
“We then employed a mouse model with lower TBX1 expression,” explains lead author Noriko Funato, “which resulted in distinct alterations to the morphology at the base of the skull and premature hardening of a cartilage joint where the bones fuse together, restricting the growth ability of the skull.” The changes in the Tbx1-knockout mice were reminiscent of the known basicranial morphology of Neanderthals.
These morphological abnormalities are also seen in human genetic diseases linked to decreased TBX1 gene dosage, such as DiGeorge syndrome and velocardiofacial syndrome, highlighting the importance of this genetic variant in the evolution of our distinct skull base morphology.
The discovery of this chromosomal variant sheds light on human evolution while also providing insight into prevalent genetic diseases associated with reduced expression of the TBX1 gene, paving the door for better understanding and therapy of these conditions.