In many parts of the world, there is a significant unmet need for clean water. A prototype of a new hydrogel tablet developed by researchers at the University of Texas at Austin can disinfect a liter of river water in one hour.
The most common method of disinfecting water is to boil it, but this takes a lot of time and energy, which may not be readily available in developing countries. Scientists are addressing the issue with devices such as solar stills, graphene filters, and automatic chlorine dispensers, but these are often inefficient or require energy.
According to some estimates, one-third of the world’s population lacks access to safe drinking water, and half of the population may live in water-stressed areas by 2025. Finding a solution to this problem has the potential to save and improve the lives of millions of people, and it is a top priority for scientists and engineers all over the world.
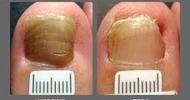
Scientists and engineers have created a hydrogel tablet that can rapidly purify contaminated water. One tablet can disinfect a liter of river water and make it suitable for drinking in an hour or less.
The University of Texas at Austin scientists and engineers have developed a hydrogel tablet that can rapidly purify contaminated water. In an hour or less, one tablet can disinfect a liter of river water and make it safe to drink. The researchers devised a method for the new study that should be relatively simple and require no energy to run. It’s a hydrogel tablet that can be dropped into a container of water and kills 99.999 percent of bacteria in about an hour. After that, the hydrogel can be removed, leaving no residue or chemicals behind.
“Our multifunctional hydrogel can make a significant difference in mitigating global water scarcity because it is simple to use, highly efficient, and potentially scalable up to mass production,” said Guihua Yu, an associate professor in the Walker Department of Mechanical Engineering at the Cockrell School of Engineering and the Texas Materials Institute.
Yu and his colleagues recently published their findings in Advanced Materials.
Today, the most common method of purifying water is to boil or pasteurize it. But that requires a lot of energy, as well as a lot of time and effort. That isn’t feasible for people in parts of the world where these processes aren’t available.

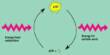
The special hydrogels produce hydrogen peroxide to neutralize bacteria with a 99.999 percent efficiency rate. Hydrogen peroxide interacts with activated carbon particles to attack and disrupt the metabolism of bacteria’s essential cell components.
The process requires no energy and produces no harmful byproducts. The hydrogels are easily removed and leave no residue behind. The tablets work by producing hydrogen peroxide, which, when combined with activated carbon particles, kills bacteria by interfering with their metabolism. According to the team, no harmful byproducts are produced during the process.
In addition to purifying water on their own, the hydrogels could improve a process that has been around for thousands of years: solar distillation, which uses sunlight to separate water from harmful contaminants via vaporization.
Biofouling, the accumulation of microorganisms on equipment that causes it to malfunction, is a common problem in solar distillation systems. The bacteria-killing hydrogels can prevent this from happening.
“A highly vigilant graduate student, Youhong Guo, discovered these hydrogels unexpectedly while doing something else,” said Keith Johnston, a professor in the McKetta Department of Chemical Engineering who co-led the project.
The team is working to improve the hydrogels by increasing the number of pathogens and viruses that they can neutralize in water. Furthermore, the team is in the process of commercializing several prototypes.
According to the researchers, scaling up the hydrogels would be simple. The materials used to make them are inexpensive, and the synthesis processes are simple and will remain so at large scales. They can also easily control the shape and size of the hydrogels, making them versatile for a variety of applications.
The paper’s first author is Youhong Guo, a graduate student in Yu’s lab. Christopher Dundas, a chemical engineering graduate student, and Xingyi Zhou, a mechanical engineering graduate student, were also members of the team. The Energy Institute at UT Austin and the Dreyfus Foundation provided funding for the study.v