Introduction:
An adhesive, or glue, is a mixture in a liquid or semi-liquid state that adheres or bonds items together. Adhesives may come from either natural or synthetic sources. The types of materials that can be bonded are vast but they are especially useful for bonding thin materials. Adhesives cure (harden) by either evaporating a solvent or by chemical reactions that occur between two or more constituents.
Adhesives are advantageous for joining thin or dissimilar materials, minimizing weight, and when a vibration dampening joint is needed. A disadvantage to adhesives is that they do not form an instantaneous joint, unlike most other joining processes, because the adhesive needs time to cure.
The earliest known date for a simple glue is 200,000 BC and for a compound glue 70,000 BC
History
The oldest known adhesive, dated to approximately 200,000 BC, is from spear stone flakes glued to a wood with birch-bark-tar, which was found in central Italy. The use of compound glues to haft stone spears into wood dates back to round 70,000 BC. Evidence for this has been found in Sibudu Cave, South Africa and the compound glues used were made from plant gum and red ochre. The Tyrolean Iceman had weapons fixed together with the aid of glue.
6000-year-old ceramics show evidence of adhesives based upon animal glues made by rendering animal products such as horse teeth. During the times of Babylonia, tar-like glue was used for gluing statues. The Egyptians made much use of animal glues to adhere furniture, ivory, and papyrus. The Mongols also used adhesives to make their short bows, and the Native Americans of the eastern United States used a mixture of spruce gum and fat as adhesives to fashion waterproof seams in their birchbark canoes
In medieval Europe, egg whites were used as glue to decorate parchments with gold leaf. The first actual glue factory was founded in Holland in the early 18th century. In the 1750s, the English introduced fish glue. As the modern world evolved, several other patented materials, such as bones, starch, fish skins and isinglass, and casein, were introduced as alternative materials for glue manufacture. Modern glues have improved flexibility, toughness, curing rate, and chemical resistance.
In the late 19th century in Switzerland, casein was first used as a wood glue. Today, it is seen to be used to glue grocery bags.
Historical Development of Adhesives and Adhesive Bonding
The history of adhesive is closely related to the history of human kind. Although most of these materials have been subject to vast changes, others have been changed very little over time. As new materials are developed, a review of the history of uses can lead one to see where they might be applied to improve old applications, and sometimes to satisfy requirements of entirely new applications.
Modern Adhesive
From the earliest days, the materials that we, later called cements, glues, gum, mucilage, resins, pastes, and finally adhesives were used interchangeably.
Historically, the first thermoplastic synthetic adhesive was the cellulose ester, cellulose nitrate, often called nitrocellulose, and it is still one of the most important.
There is little agreement in the literature about the dates when various adhesive were first development or used in a specific application. This is due to simultaneous developments in many parts of the world and the fact that references in the literature are almost exclusive from the more developed countries.
Synthetic rubber, a dimethylbutadine, was developed as a substitute for natural rubber and saw limited use as an adhesive but today SBR adhesives are the most important adhesive in the USA. Neoprene rubber adhesive are available as both thermoplastic and cross-linking systems in both solvent and emulsion formulation. Neoprene rubber is the major base resin for contact adhesives. Most of the thermoplastic resins were soluble in organic solvents and were used as solvents adhesives for different materials. PVC, a thermoplastic developed in 1927, is used today in solvent formulation to bond PVC article such as coated fabrics, films, foams and pipe. PVC was used as a solvent-based adhesive in the 1930s, and later as a hot melt, but was not of commercial importance until its introduction in the 1940s as an emulsion adhesive. Today, in emulsion form as a white glue, it is the most widely thermoplastic adhesive worldwide. Today acrylic adhesive appear in many forms; as both pressure sensitive and non-pressure sensitive formulation in organic solvents and emulsion forms.
Ideal Properties of Adhesive
- High cohesive strength.
- High adhesive strength with wide range of materials.
- High resistance to creep up to 60°C at least, but capable of heat activation at higher temperature.
PU80°C-90°C
- Bond strength should not be reduces by grease, plasticizer, perspiration water or other substances to which it might normally be exposed.
- High resistance to ageing.
- High flexibility and resistance to flex cracking.
- Easily applicable by hand or machine.
- Controllable viscosity and good wetting and penetrating properties.
- High enough solid content.
- Controllable drying rate to suit working environment.
- Long tack life for maximum versatility in work organization.
- Suitable green strength and rate of set up low green strength for socking and stiffener attachment to allow repositioning. High for folding and lasting to prevent ‘spring back’.
- Should be non flammable and non-toxic.
- Excess adhesive should be easily removable from visible part of shoe, having no marks or stain.
- Long shelf life, not too sensitive to storage temperature.
- Should be economic to use.
- No shrinkage or drying.
Adhesion
Adhesion is any attraction process between dissimilar molecular species that can potentially bring them in “direct contact”. By contrast, cohesion takes place between similar molecules.
Adhesion is the tendency of dissimilar particles or surfaces to cling to one another (cohesion refers to the tendency of similar or identical particles/surfaces to cling to one another). The forces that cause adhesion and cohesion can be divided into several different types. The intermolecular forces responsible for the function of various kinds of stickers and sticky tape fall into the categories of chemical adhesion, dispersive adhesion, and diffusive adhesion. In addition to the cumulative magnitudes of these intermolecular forces, there are certain emergent mechanical effects that will also be discussed at the end of the article.
Principle of Adhesion
At the time of application the adhesive must be a liquid, as this enables it to make intimate molecular contact with the adherents; that is it must wet the surfaces. It must then harden (cure) to a cohesive solid, pressure sensitive adhesives are an exception in that they do not harder, but remain permanently sticky.
In simple terms, materials are held together by attractions (‘magnetic forces’) between the atoms and molecules from which they are built up. The same forces might reasonably be expected to cause materials brought into contact to stick together.
Unfortunately, just as a toy magnet will only attract a pin at close range, intermolecular forces only operate over very short distances (one millionth of a mm!), so the necessary close contact is never achieved even with highly-polished surfaces.
Mechanisms of Adhesion
There are two main mechanisms by which an adhesive (cement) sticks to a material. These are referred to as Mechanical Adhesion and Specific Adhesion.
Mechanical Adhesion
This is the more common of the two, being effective to some extent in almost all examples of bonding. Many materials have visibly rough surfaces, or have a fibrous structure which is porous, close examination shows that even the smoothest surfaces contain microscopic pores. When adhesive is applied in liquid form to the surface, some of it flows into these pores. After drying, the adhesive layer will be “keyed1 to the material surface rather like two pieces of a jigsaw puzzle are joined together.
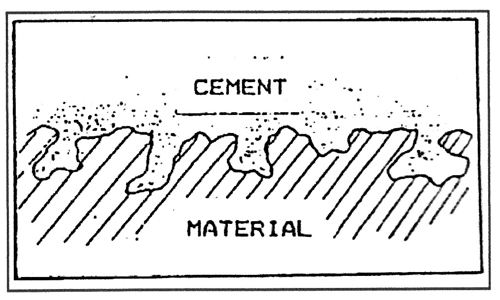
The effectiveness of the bond will depend on the strength of the material, and on the size, depth and shape of the pores. Deep and undercut pores will lead to stronger bonds than shallow indentations.
Chemical adhesion
On a molecular scale, bonding occurs when adhesive molecules diffuse into and become intertwined with the molecules in the material surface. For this to happen the attractive forces between adhesive molecules and material molecules must be at least as strong as the attraction of adhesive molecules for each other. I.e. the adhesive is more specific in what it will bond to.
The diffusion process is helped by the presence of solvents in the adhesive which swell or partially dissolve the material surface. Heat performs a similar function when using hot melt cements, and when causing one cement film to coalesce with another after reactivation.
In another type of specific adhesion the molecules in the cement become bound by strong chemical bonds to molecules in the material. For this to happen there must be specific chemical structures present in the two bonding surfaces. Frequently the material surface is made chemically reactive to the cement just before spreading by applying a special primer. An example is the use of a halogenating agent on thermoplastic rubber before applying PU cement.
Two materials may form a compound at the join. The strongest joins are where atoms of the two materials swap (ionic bonding) or share (covalent bonding) outer electrons. A weaker bond is formed if oxygen, nitrogen or fluorine atoms of the two materials share a hydrogen nucleus (hydrogen bonding).
Cohesion causes water to form drops, surface tension causes them to be nearly spherical, and adhesion keeps the drops in place. Water droplets are flatter on a Hibiscus flower which shows better adhesion. Five mechanisms have been proposed to explain why one material sticks to another.
Dispersive adhesion
In dispersive adhesion, also known as adsorption, two materials are held together by van der Waals forces: the attraction between two molecules, each of which has a regions of positive and negative charge. In the simple case, such molecules are therefore polar with respect to average charge density, although in larger or more complex molecules, there may be multiple “poles” or regions of greater positive or negative charge. These positive and negative poles may be a permanent property of a molecule (Keesom forces) or a transient effect which can occur in any molecule, as the random movement of electrons within the molecules may result in a temporary concentration of electrons in one region (London forces).
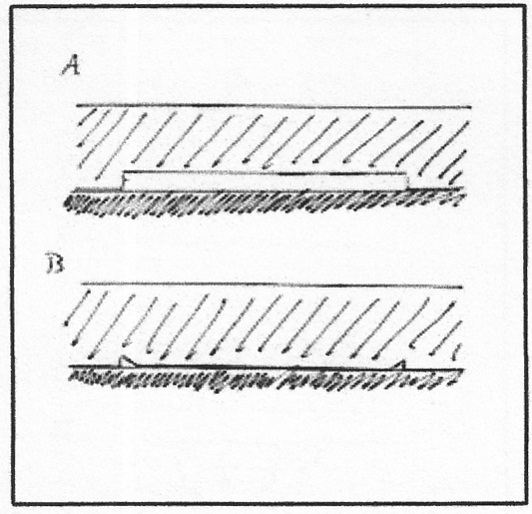
Electrostatic Adhesion
Some conducting materials may pass electrons to form a difference in electrical charge at the join. This results in a structure similar to a capacitor and creates an attractive electrostatic force between the materials:
Diffusive Adhesion
Some materials may merge at the joint by diffusion. This may occur when the molecules of both materials are mobile and soluble in each other. This would be particularly effective with polymer chains where one end of the molecule diffuses into the other material. It is also the mechanism involved in sintering. When metal or ceramic.
ADHESIVE CONCEPTS, TERMINOLOGY DEFINITIONS AND GLOSSARY OF TERMS
Adherend
These are materials used for attaching by means of an adhesive. Shoe uppers, soles, toe puff etc. are a few examples for adherent or substrates.
Accelerator
A substance which speeds up a chemical reaction during the curing up of an adhesive bond.
Filler
A material responsible for improving a few properties of an adhesives and also bringing down the cost.
Retarder
A chemical to an adhesive to slow down the chemical reaction.
Inhibitor
These chemicals in an adhesive prolong the storage life.
Hardener
Chemicals when added to an adhesive promote storage life.
Modifier
An ingredient chemically inert but changes the property of an adhesive without taking part in the reaction. These may be fillers thinners, plasticizers, stabilizers or wetting agents.
Plasticizer
Plasticizer in an adhesive reduces the viscosity & brings down the temperature required for heat reactivation, increases the flexibility of an adhesive.
Tackifier
Tackifier resins are of low molar mass & have a higher glass transition temperature than the base polymer. They used in pressure sensitive adhesive & hot-melt adhesive.
Thinner
Also known as deluent or extenders, a volatyl liquid added to an adhesive to modify the consistency or working properties.
Stabilizer
A substance which increases the resistance to light, heat, radiation & so on.
Shelf Life
The expected storage life in unopened containers. It is determined by the stability of the adhesive at the storage temperature to chemical and physical changes
With 1 -part cements it refers to the maximum usable life after opening the container. With solvent cements it is almost impossible to reseal containers efficiently and solvent will be lost by evaporation more or less slowly depending on volatility. It can of course be replaced, but if this is not done the liquid becomes too thick and lumpy to reconstitute effectively. Rate of solvent loss when a container is in use depends on its dimensions. In general the pot life will be longer if the container is narrow and deep rather than wide and shallow. There are applicators available designed to reduce evaporation to a minimum. Some adhesives are affected by exposure to atmospheric moisture.
With 2-part cements, pot life refers to the maximum usable life after mixing the two components. The mixing initiates a chemical reaction which results in the adhesive eventually becoming solid. Rate of reaction depends on the chemical nature and amount of ‘hardener’ used, temperature, and total amount mixed. In general the greater the proportion of hardener and the higher the temperature the faster is the reaction and the shorter the pot life. The reaction produces heat. The greater the amount mixed the harder it is for the heat to escape and the higher the temperature rises. Consequently the faster the reaction proceeds, the shorter the pot life.
Drying time
This is the time required for a spread cement film to lose sufficient water or solvent for a successful bond to be made. It applies mainly to water or solvent-based cements using a two-way dry stick method. The times are normally given for natural drying (at room temperature) but can be reduced considerably by passing coated components through hot air tunnels. The higher the temperature and the better the air circulation the faster the drying rate. Drying times are shorter for films on porous materials like leather and fabrics than on non-porous materials like PVC, VR, TR etc. The thicker the film the longer the time Failure to allow sufficient drying time will result in bonds with poor green strength and likely to ‘spring back’. With hot melt cements, there is no drying time because there is no solvent. For liquid curing adhesives, drying time refers to the time taken for the film to develop sufficient tackiness (spotting tack) for efficient bonding using a minimum press time. As a general rule the shorter the drying time the shorter is the pot life but the more cost-effective is the bonding process.
Pressure-sensitive life
This is the time after drying that a cement film retains enough tackiness for a bond to be made by applying pressure alone to the components. Dry tack of many adhesives can be increased by the addition of various resins. The pressure-sensitive life of cement films is reduced by the presence of dust in the air. Much of the cements have no pressure sensitivity once dry.
Tack retention tame reactivation life or tack life
This refers to the maximum time after drying that a cement film can be successfully reactivated by heat for good bonding. For some time after the pressure-sensitive life of an open film has expired, tackiness can be recovered (or generated) by heating the surface of the film to the correct activation temperature. Tack life is reduced by dust and ageing, and can sometimes be extended wiping the film.
Open time
This term is ‘used by some people to mean tack life. Here it refers to the actual time which elapses between applying the adhesive and making the bond in the shoe factory. It depends on the work organization. Maximum open time should not exceed the tack life of the cement.
Spotting tack
This refers to bond strength developed when two cement films are lightly pressed together. It should be sufficient for the components to remain in place while they are transferred to the press; but not so high that the components cannot easily be re-positioned if necessary before applying foil pressure.
This is the minimum time for which the components must be under pressure in the bonding press. It is determined by the rate of set-up.
Bonding pressure
This is the recommended pressure to be applied to the particular combination of materials and adhesive in. the press to achieve the best results. It controlled by the coalescent properties of the cement and the compressive properties of the materials e g. Expanded soling should not be subjected to as high a pressure as harder solid soling because of the risk of permanent deformation. Up to a point, the higher the press.
Importance/Use of Adhesives in Footwear Making
Adhesives form an important part of footwear chemicals in general and shoe chemicals in particular. Adhesives used in footwear are for two purposes-Temporary and Permanent joint. Most of the footwear require temporary joint while assembling; and they are stitched later to be permanent for the end use. So water based latex and rubber solutions serve the best result for this purpose. Other kind of products is made only by using adhesives; so permanent joint is achieved with it. Solvent based polychloroprene and polyurethane serve the best result in this respect as they are permanent types.
Natural rubber makes strong adhesives which have proved their suitability time and again for shoe trade operations; but of the synthetic materials can give as strong an adhesion and are not as susceptible to be attacked by grease and oxidizing agents.
Processes using Adhesive in Footwear Manufacturing: Folding Operations:
Three main types of cements are used. These are the latex rubber, solvent rubber, hot melt cement.
Backing Operations
Again the three systems are available; they are the latex cement, Rubber cement and thermoplastic backer.
Heel covering
The operations of heel covering is usually conducted by putting coating the heel blocks with a latex cement and the cover with a latex or solvent rubber cement.
Insole Lip Cementing
This operation is carried out in the BUSMC Model’C Economy Insole Lip Cementing machine.
In this machine latex cement is applied by roller to the insole Lips.
Attaching rib-welted Insoles
In the prime process a moderately heavy cotton tape 7/8″ wide is cemented to the insole with a neoprene or natural rubber latex cement.
Unishank cementing
Latex cement are used for this operation in the BUSMC No. 2 Unishank cementing machine.
Counter pasting
For this operation the BUSMC counter pasting machine Model ‘£’ is used with compounded latex cement.
- Cement lasting
- Toe lasting-this operation is conducted in the bed laster is carried out by the use of latex cement.
- Side lasting
- Latex cement are invariably used.
- Sole laying
Sole laying should be carried out using latex cement or natural rubber cement.
Cement sole attaching (BUSMC Method)
All types of shoe outsoles can be attached by cementing processes, but different materials require the correct type of cement and the right method is used. Many millions of pairs of shoes have been manufactured all over the world with neoprene cement performing the service of sticking the outsole. Recently, polyurethane are increasingly used for their performance though they the are more costlier than neoprene.
Crepe attachment
Crepe outer soles may be attached directly to the upper using the combination of latex adhesive and rubber cement.
Attaching vulcanized rubber soles
Forms of attachments are very similar to those employed for crepe sole, but instead of activity cement it is often essential to have a vulcanizing rubber solution.
Attachment of microcellular outsoles
Natural rubber is used very effectively for this purpose.
The attachment of resin rubber outsoles
In using resin rubber the most suitable cements are the neoprene types.
Pasting in socks
Latex cement are the most satisfactory for sticking socks.
Main Classification of Adhesives
1. Water based adhesive.
(a) Natural rubber latex.
(b) Synthetic lattices
(c) Vegetable paste
2. Solvent based types adhesive.
(a) Rubber solution
(b) Polychloroprene solution
(c) Poly Urethane
(d) Nitrocellulose adhesives.
3. Hot melt adhesive
4. Heat fusible coatings
5. Pressure sensitive.
6. Cyanoacrylates (Super Glue)
There are mainly two types of adhesive
a. Water based adhesive
b. Solvent based adhesive
Water Based Adhesive
Natural Rubber Latex
Natural rubber is a product existing as a milky substance known as latex. It is obtained from “Heveabrasiliensis”. Latex is a colloidal dispersion of rubber particle in water.
Basically, latexas tapped from the rubber tree. But usually compounded with resins to improve tack.Contains ammonia for stability.
Properties
1. Nonflammable
2. Good initial grab
3. Clean in use
4. Spray able
5. Low plasticizer resistance
6. Versatile bonding method
7. Poor heat resistance.
- Dry stick.
- Wet stick.
Dry stick
Apply to both surfaces allow to dry, make immediate bond by placing surfaces together using self adhesive properties.
Wet Stick
Only needs one coat on one of the sticking surfaces, the other surface applied to the wet coat and allowed to dry in contact.
Uses
1. Fitting, Laminating, Folding
2. Toe puff attaching
3. Stiffener attaching
4. Insole binding
5. Socking
6. Heel covering
2. Synthetics Latices
It is obtained from emulsion or dispersion of synthetics polymer such as polyvinyle acetate (PVA), acrylate, polycholoprene or polyurethane (PU) in water.
Properties
1. Nonflammable
2. Relatively poor wet “grab”
3. Superior plasticizer resistance.
Uses
1. Socking
2. Toe puff attaching
3. Stiffener attaching
4. Heel covering
3. Vegetable Paste
It is made from starch that is a derivative of starch.
Properties
1. Non flammable
2. Low bond strength
3. Soften by water
Uses
Box leveling
Rubber Solution
It is prepared by milling latex together with the compounded rubber and dissolving the same in a solvent such as benzene or gasoline. A resin tackifier is also used.
Properties
1. Generally flammable
2. Good tack but not high strength
3. Limited plasticizer resistance
4. Moderate heat resistance
5. Sensitive to oil and organic solvents
6. Petroleum solvent based solution unlikely to damage material
Uses
1. Upper to lining attachment
2. Sock lining to insole attachment
3. Temporary bonding for edge folding
2. Neoprene
Polychloroprene is a synthetic elastomer with many of the properties of natural rubber. These are prepared in number of grades depending on the crystallization rate.
Ingradients
1. Resins
2. Anti-oxidants
3. Fillers.
4. Solvent.
5. Accelerator.
6. Cross linking agent.
Properties
1. High bond strength
2. Good grab
3. Limited plasticizer resistance
4. Easy handing
5. Can use with brush or spray
6. Difficult to remove if materials are contaminated
7. Long tack life
Uses
1. Fitting, laminating
2. Insole laminating
3. Insole rib attaching
4. Rubber and leather sole laying
5. Lasting
6. Heel covering
3. Polyurethane
Polyurethane is produced when a di-isocyanate having two isocyanate groups is reacted with a diol havig two hydroxyl groups.
Ingradients
1. Polyols.
2. Di-issocyanate.
3. Curing agent.
Properties
1. Flammable
2. Strong bond with most martial
3. Superior plasticizer resistance
4. Grease and oil resistance
5. High green strength but at least 48 hours needed to reach full strength
6. Difficult to remove if materials are contaminated
Uses
1. Sock attaching
2. Sole attaching
3. Hand lasting
Description of the Different Types of Adhesives
Natural adhesives
Natural adhesives are made from organic sources such as vegetable matter, starch (dextrin), natural resins or from animals e.g. casein or animal glue. They are often referred to as bioadhesives. One example is a simple paste made by cooking flour in water. Animal glues are traditionally used in bookbinding, wood joining, and many other areas but now are largely replaced by synthetic glues. Casein are mainly used in glass bottle labelling. Starch based adhesives are used in corrugated board production and paper sack production, paper tube winding, wall paper adhesives. Another form of natural adhesive is blood albumen (made from protein component of blood), which is used in the plywood industry. Animal glue remains the preferred glue of the luthier. Casein based glues are made by precipitating casein from milk protein using the acetic acid from vinegar. This forms curds, which are neutralized with a base, such as sodium bicarbonate (baking soda), to cause them to unclump and become a thicker plastic-like substance.
Synthetic adhesives
Synthetic adhesives are based on elastomers, thermoplastics, emulsions, and thermosets. Examples of thermosetting adhesives are: epoxy, polyurethane, cyanoacrylate and acrylic polymers.
Drying adhesives
These adhesives are a mixture of ingredients (typically polymers) dissolved in a solvent. Glues such as white glue, and rubber cements are members of the drying adhesive family. As the solvent evaporates, the adhesive hardens. Depending on the chemical composition of the adhesive, they will adhere to different materials to greater or lesser degrees. These adhesives are typically weak and are used for household applications, g for use by small children are now made non-toxic.
Contact adhesives
Contact adhesives are used in strong bonds with high shear-resistance like laminates, such as bonding Formica to a wooden counter, and in footwear, as in attaching outsoles to uppers.
Natural rubber and polychloroprene (Neoprene) are commonly used contact adhesives. Both of these elastomers undergo strain crystallization.
Contact adhesives must be applied to both surfaces and allowed some time to dry before the two surfaces are pushed together. Some contact adhesives require as long as 24 hours to dry before the surfaces are to be held together. Once the surfaces are pushed together, the bond forms very quickly. It is usually not necessary to apply pressure for a long time, so there is less need for clamps.
Hot melt adhesives
A hot glue gun loaded with a glue stick. Hot melt adhesive (or hot glue) is a form of thermoplastic adhesive that is commonly supplied in solid sticks designed to be melted in a special gun. The glue comes in cylinders of various lengths, and is pushed into an electric hot glue gun. The gun contains a heating element to melt the plastic glue, which operates all the time the gun is plugged in. Squeezing the trigger pushes the stick through the heating element, ejecting molten glue which is initially hot enough to burn and blister skin. The glue is tacky when hot, but hardens and stops being sticky in a few seconds-a minute at most.
Animal glue which is applied hot has been used in woodworking since the time of the Ancient Egyptians. It is heated in a glue pot and applied with a brush.
However, the most common application for hot melt glue is industrial. Hot melt can be found in many products that we purchase each day.
Hot Melt Adhesives Importance of hot melt adhesives has grown steadily in shoe construction as an alternate bonding, method to solvent and solvent-free adhesives specifically in shoe lasting. These are of cent percent solid content and are free from solvents. The availability of a variety of polymers with a broad spectrum of properties tailored for a specific end use is an added advantage. To-day hot melt formulations are available for temporary as well as permanent attachment in shoe manufacture.
Hot Melt Adhesives are usually supplied to footwear industry in the form of granules and rods (in coils). When these adhesives are heated to higher temperatures, they melt rapidly into a fluid, flow freely on bonding sites, wet the surfaces, quickly cool, solidify to a crystalline solid and thereby adhere to give good bond strength between the two surfaces.
Base Polymers of hot melt adhesives used in shoe construction usually consists of polyamides, polyesters, ethylene- vinyl acetate and cellulosic polymers.
Polyesters Polyesters are synthetic resins having ester linkages in the main chain. These are produced by the reaction between dihydric alcohols and dicarboxylic acids.
Preparation of adhesive In the preparation of polyester hot melt adhesive, methyl esters of terephthalic acid and isophthalic acid are mixed with 1,4 -butanediol and are heated to about 140°C in an atmosphere of nitrogen, lead peroxide.
Is added, stirred and heated at 140°C. Displaced methyl alcohol is collected. The temperature is increased to 220°C to distill butanediol and the heating is continued to, 600C. The molten mass is extruded as a rod cement througVh a die into cold water where it solidifies, then rolled on to pools and ready for use.
Polyester adhesive for shoe lasting At the time, of lasting, the adhesive rod is heated 10 a temperature above the melting point and deposited on the substrate The adhesive softens at 180°C melts at 200°C and when deposited on the substrate has a temperature ‘of 120°C. At the same time the wiper plates pushes the upper on to the insole with the molten adhesive in between. The molten copolyester has low surface tension and viscosity which is responsible for the molten adhesive to wet flow and spread on the surface, in between the substrates. The molten adhesive is super cooled, solidifies into a oriented crystalline state forming a very strong bond between the surfaces.
Adhesive for out sole attachment hot melt adhesives are also used for the sole attachment to the lasted uppers. Depending upon the composition and concentration of various ingredients the adhesive is intended, either for leather soles or -m-leather soles the adhesive should have low viscosity set rapidly and activated at a low temperature. Low viscosity enables the behaviour of flowing adhesive to wet the surfaces lasted margin of uppers). Low sole activation temperature enables the operator to handle the soles, safely without burning his Inrgers. The adhesive should set rapidly in between the upper end the sole. Rapid setting time prevents the sole lagging, away from the upper after the shoe is taken out from sole, altMChing press.
Pressure sensitive adhesives
Pressure sensitive adhesives (PSA) form a bond by the application of light pressure. They are designed with a balance between flow and resistance to flow. The bond forms because the adhesive is soft enough to flow (i.e. “wet”) the adherend. The bond has strength because the adhesive is hard enough to resist flow when stress is applied to the bond. Once the adhesive and the adherend are in close proximity, molecular interactions, such as van der Waals forces, become involved in the bond, contributing significantly to its ultimate strength.
Pressure sensitive adhesives are manufactured with either a liquid carrier or in 100% solid form. Articles are made from liquid PSAs by coating the adhesive and drying off the solvent or water carrier. They may be further heated to initiate a cross-linking reaction and increase molecular weight. 100% solid PSAs may be low viscosity polymers that are coated and then reacted with radiation to increase molecular weight and form the adhesive; or they may be high viscosity materials that are heated to reduce viscosity enough to allow coating, and then cooled to their final form. Major raw material for PSA’s are acrylate based polymers.
Thermal adhesive
Thermal adhesive is a type of thermally conductive glue used for electronic components and heatsinks. Thermal adhesive is similar to thermal paste in that it is used for transferring heat from one surface to another, it usually consists of two separate parts that are mixed together and applied to the surfaces to be bonded. This is commonly used to bond heatsinks to motherboard chipsets and video card processors where there are no mounting holes to clamp a heatsink down.
Thermal adhesive is usually applied by the manufacturer of the part to which it is applied, although it is for sale to the public.
Most Commonly Used Adhesives
These types of adhesives are mostly used in footwear industry.
Natural Rubber/Latex
Natural rubber is a product existing as a milky substance known as LATEX obtained from “Heveabrasiliensis”. Latex is a collodial dispersion of rubber particles (hydrocarbons) in water. In addition there are traces of proteins, fatty acids, resin like substances and mineral salts acting as emulsifiers, antioxidants, accelerators providing adhesive properties.
Adhesives of natural rubber are prepared by
– dispersing latex in water (water based latex) mixing in suitable solvents
– by grafting with a polymer.
Water Based Latex Natural rubber is centrifuged to get 60% concentrated latex. A solution of ammonia in water is prepared and added. The concentration of ammonia depends on the material to be attached. Depending upon the end use, casein and or phenolic resins are also added. Such adhesives are used for outsole channel closing of welted constructions, outsole attachment to the upper where the sole is stitched to the upper, top line binding, upper to lining attachment and so on.
Non-curing cement for temporary bonding may consists of natural rubber latex, ammonia caseinate solution and zinc oxide solution of 60%, 10% and 60% concentrations respectively.
Rubber solution
Rubber solution is prepared by milling latex, together with the compounded rubber (crepe or smoaked sheets) and dissolving the same in a solvent such as benzene or gasoline. A resin tackifier is also used. Rubber solution is used for establishing temporary bonding for edge folding, upper to lining attachment and sock lining attachment to insole and so on.
Modified rubber
Latex is modified by grafting with acrylic monomers (e g,.) methyl methaciylate. This graft copolymer is lightly milled and dissolved in solvents. This formulation could be tried for establishing good bond between a variety of soles with leather uppers.
Formulations for the establishment of a permanent bond are ported. Various ingredients for such a formulation may -i of natural rubber latex with 60% solid content, n of potassium hydroxide, zinc oxide, sulphur, sulphur an antioxidant, resin emulsion and ammonia casemate solution.
Natural rubber
Adhesive which has curing properties is prepared from natural latex 60% (solids), 10% potassium hydroxide solution, 60% zinc oxide, 6% sulphur dispersion, antioxidant, 40% resin emulsion and 10% ammonia caseinate solution.
Latex adhesive
Reducing the solvent content or eliminate the solvent completely as suggested by Arendth. These adhesives use plasticizers which perform the properties similar to the solvents, provides the viscosity response, improve wetting and normally affect adhesive application properties favourably. The plasticizers are Benzoate ester plasticizers.
Neoprene Adhesive
Polychloroprene is a synthetic elastomer with many of the properties of natural rubber. These are prepared in number of grades depending on the crystallisation rate. Adhesives of polychloroprene also known as neoprene adhesives and have the advantages of
– film flexibility
– high bond strength,
– easy handling
– bonds quite a number of materials
– application through a brush or spraying -resistant to deterioration caused by chemicals,
– oils and heat hi addition to the above advantages the adhesives
– readily crystallises even at room temperature
– posses high polarity
– dissolve in a number of solvents
– can control tack retention time ingredients used in polychloroprene adhesives are
– resins
– antioxidants
– solvents
– fillers
– accelerators
– crosslinking agents.
Pqlyurethan1e Adhesive
The chemistry of POLYURETHANES has revolutionised the science and technology of shoe manufacture as an alternate material to the natural product namely leather. The contribution of polyurethane to shoe industry is tremendous. To day one is able to fabricate a full shoe out of polyurethane. Polyurethanes are used for shoe uppers, shoe soles, adhesives and shoe finishes.
Polyurethane
Polyurethane (PU) is produced when a diisocyanate having two isocyanate groups is reacted with a diol having two hydroxyl groups.
Polyurethane adhesives:
Various ingredients used in the formulation of PU adhesive are
(a) Polyols having a molecular weight more than 1000. The polyols may be a polyether, polyester or polybutadiene based.
(b) Diisocyanate: This is either toluene diisocynate (TDI) or methylene bis (4.4- phenyl isocyanate) (MDI)
(c) Curing agent: The curing agent may be a short chain did or trio for MDI and an aromatic diaminer for TDI.
Polyols
Polyols are classified into polyether diols, polyester diols and polycarbonate diols.
Polyether polyols are addition products derived from cyclic ethers (ethylene oxide or propylene oxide), pylene oxide), using proper initiators such as water, alcohols or amines. Polyester polyols are produced by reacting di- functional alcohol or tri-functional alcohol with carboxylic acids.
Polyols are hygroscopic, because of this the ingredients used in adhesive manufacture are kept dry. If necessary, moisture absorbing chemicals or chemicals to prevent hydrolysis of polyols are added.