There is a critical need to image these processes noninvasively in order to identify molecular activities that define the early stages of disease progression. The magnetosome is an ideal structure for fine-tuning cellular and molecular magnetic resonance imaging (MRI).
A research team led by Prof. Wang Junfeng of the Chinese Academy of Sciences’ Hefei Institutes of Physical Science (HFIPS) biomimetically synthesized soft ferromagnetic nanoparticles with high magnetic targeting and tumor tissue penetration based on the biomineralization mechanism of a natural “biocompass”—magnetotactic bacteria—in a study published in PNAS.
Targeted delivery of anti-tumor drugs can significantly improve efficacy while reducing toxicity. Because of the limitations of the tumor microenvironment’s complexity, the average tumor targeting efficiency of nanodrugs is less than 1%, constituting one of the bottlenecks of tumor therapy.
The magnetosome-like nanoparticles synthesized with this method performed excellently. The DSPE-mPEG–coated magnetosome-like magnetic nanoparticles penetrated the lesion area of a tumor mouse model.
Ma Kun
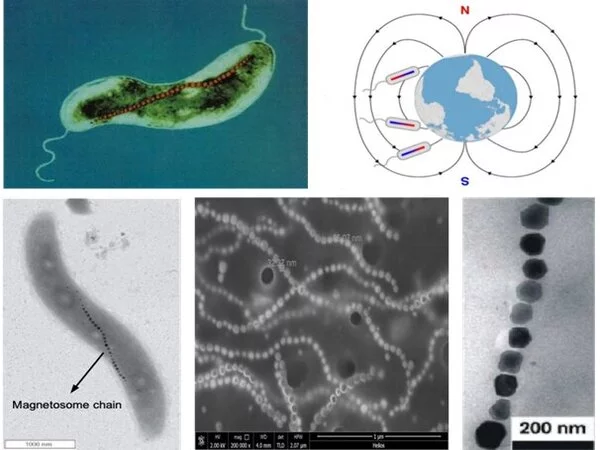
Animals such as pigeons, turtles, and lizards can use the geomagnetic field to navigate. After obtaining iron from the surrounding environment, bacteria can move directionally along the magnetic field in a geomagnetic or artificial magnetic field. Magnetosomes have a wide range of application prospects due to their obvious advantages in magnetic properties, biocompatibility, and stability.
Natural magnetosome particles, on the other hand, are easily accumulated and precipitated in the external environment, limiting their ability to penetrate the lesion area and posing the risk of deposition in blood vessels.
Precision medicine’s rise, which aims to tailor personal medical treatments and therapies as well as human well-being monitoring, is widely supported by public research policies around the world. While medical imaging such as X-rays, echography, Positron Emission Tomography (PET), and MRI (Magnetic Resonance Imaging) are used on a daily basis to obtain anatomical and functional information, subcellular and even molecular detection of prognostic and diagnostic physiological markers is attempting to reach the patient’s bedside.
The design of effective drug distribution systems in the body is an important area of research for increasing the efficacy of cancer therapies. The development of new strategies is primarily aimed at improving the stability of the drug after administration and increasing the precision of drug delivery to the destination.
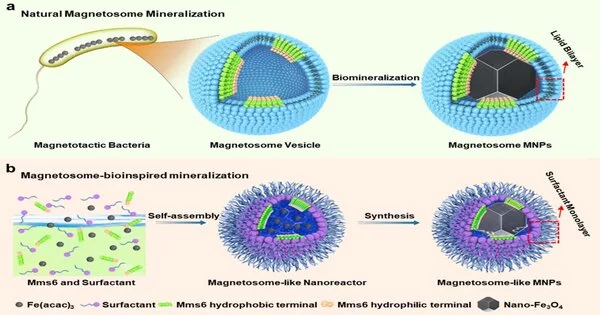
The researchers proposed a new strategy for biomimetic magnetosome synthesis in this paper. They built a magnetosome-like nanoreactor and recreated the magnetosomes’ biomineralization microenvironment in vitro.
“The magnetosome-like nanoparticles synthesized with this method performed excellently,” said Ma Kun, first author of the study, “The DSPE-mPEG–coated magnetosome-like magnetic nanoparticles penetrated the lesion area of a tumor mouse model.”
Experimental findings demonstrate the improvement by an order of magnitude in the targeting and penetrability of biomimetic magnetosomes in tumor tissues compared with other magnetic nanodrugs.
In many cases, molecular imaging necessitates the development of specific functionalized contrast agents in order to reveal molecular or cellular phenomena using a dedicated imaging modality. PET imaging is currently the most advanced modality for this purpose, with high sensitivity for biomarker detection but low spatial resolution and the use of radioactive compounds as major drawbacks.
This study not only develops a new model system for studying the biomineralization mechanism of magnetotactic bacteria in vitro, but it also provides an efficient carrier for magnetic targeting delivery of nanodrugs.