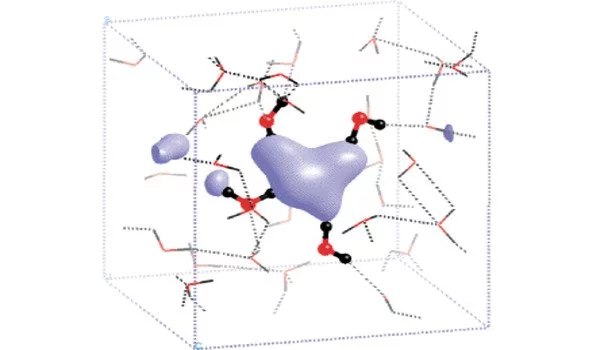
In isolation, dielectrons, which are made up of two free electrons bound together, are highly reactive and unstable. They typically exist only briefly in high-energy environments like particle accelerators or under certain astrophysical conditions. However, when they interact with solvent molecules in a liquid or solution, their behavior and stability may change.
Scientists have many hypotheses about solvated dielectrons, but they have never been directly observed. They are described as a pair of dissolved electrons in liquids such as water or liquid ammonia. A cavity forms in the liquid to make room for the electrons, which the two electrons occupy.
An international research team led by Dr. Sebastian Hartweg, now at the Institute of Physics at the University of Freiburg, and Prof. Dr. Ruth Signorell from ETH Zurich, as well as scientists from the synchrotron SOLEIL and Auburn University (US), has discovered a process for the formation and decay of the solvated dielectron.
The consortium discovered direct evidence for the formation of these electron pairs by excitation with ultraviolet light in tiny ammonia droplets containing a single sodium atom in experiments at the synchrotron SOLEIL (DESIRS beamline). The findings were recently published in the journal Science.
These are intriguing prospects for future applications. Our work lays the groundwork for this and contributes to a better understanding of these exotic and still enigmatic solvated dielectrons.
Dr. Sebastian Hartweg
Traces of an unusual process
When dielectrons are formed in tiny ammonia droplets containing a sodium atom by excitation with ultraviolet light, they leave traces in an unusual process that scientists have now observed for the first time. During this process, one of the two electrons migrates to neighboring solvent molecules while the other is ejected.
“What’s surprising about this is that similar processes have previously been observed primarily at much higher excitation energies,” Hartweg says.
The team focused on this second electron because there could be interesting applications for it. On the one hand, the ejected electron is produced with very low kinetic energy, so it moves very slowly. On the other hand, this energy can be controlled by the irradiated UV light, which starts the whole process. Solvated dielectrons could thus serve as a good source of low-energy electrons.
Generated specifically with variable energy
Slow electrons can initiate a wide range of chemical processes. They, for example, play a role in the chain of events that leads to radiation damage in biological tissue. They are also useful in synthetic chemistry, where they act as efficient reducing agents. The ability to selectively generate slow electrons with variable energy will allow researchers to study the mechanisms of such chemical processes in greater depth in the future.
If solvated dielectrons were discovered for the first time since my knowledge cutoff, it would indicate a novel and intriguing phenomenon. This discovery has the potential to have far-reaching implications for our understanding of electron behavior in various environments, as well as for fields such as chemistry, physics, and materials science.
Furthermore, the energy made available to electrons in a controlled manner could be used to improve the efficiency of reduction reactions. “These are intriguing prospects for future applications,” says Hartweg. “Our work lays the groundwork for this and contributes to a better understanding of these exotic and still enigmatic solvated dielectrons.”