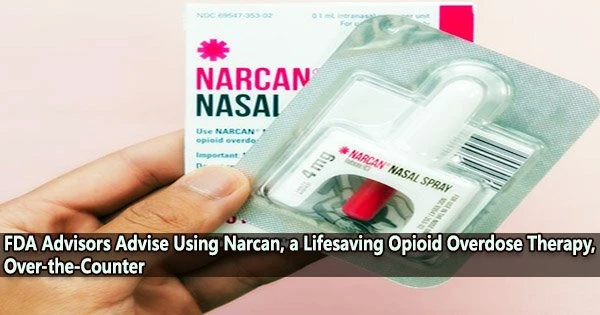
The independent advisors to the Food and Drug Administration (FDA) unanimously approved the over-the-counter use of the nasal spray Narcan to treat opioid overdoses on Wednesday (February 15, 2023). This would greatly increase access to the life-saving medication.
The drug Narcan from Emergent BioSolutions is the one used to treat opioid overdoses the most frequently. By March 29, the FDA is anticipated to decide whether to permit non-prescription purchases of the 4 milligram nasal spray. Although it usually does, the agency is not compelled to adopt the advice of its advisors.
“There is no reason to keep this as a prescription, let’s get it out there and save some lives,” said Elizabeth Coykendall, a paramedic at PM Pediatrics in Raleigh, North Carolina, and a temporary voting member of the FDA committee.
Emergent BioSolutions said Narcan would be available for the over-the-counter market by late summer if the FDA approves it next month. The company has not yet disclosed how much it would cost.
“We have been working on distribution plans with key stakeholders like retailers and government leaders,” said Matt Hartwig, a spokesperson for the company.
The majority of states have already granted general prescriptions that enable pharmacies to dispense Narcan, also known as naloxone, without the need for the patient to provide a prescription. However, more people would be able to access the medication more quickly and in more locations if the FDA approved Narcan for over-the-counter usage.
“If naloxone becomes a nonprescription product, it may be sold in many venues previously unavailable to consumers, including vending machines, convenience stores, supermarkets and big-box stores, just like other nonprescription products,” Jody Green, an official at the FDA’s nonprescription drug division, told the advisory committee Wednesday.
According to the Centers for Disease Control and Prevention, over 564,000 Americans have died from opioids since 1999, with the first wave being caused by prescription opioids, the second by heroin, and the third by fentanyl. Opioid overdose deaths spiked 17% during the Covid pandemic from about 69,000 in 2020 to nearly 81,000 in 2021.
The Trump administration first declared the opioid epidemic a public health emergency in 2017. The Biden administration has renewed the emergency declaration every 90 days since the president took office.
“Each day 187 people will die this is absolutely tragic as we think of not only the individuals themselves, but the families, the communities, the workplaces. This has profound human impact and we are all impacted from this,” Manish Vyas, senior vice president of regulatory affairs at Emergent BioSolutions, told the committee.
Dr. Scott Hadland, head of adolescent medicine at Massachusetts General Hospital, said the widespread infiltration of fentanyl into the nation’s drug supply has increased the risk of overdoses. Many people who are exposed to fentanyl take counterfeit pills that they thought were prescribed but actually contain the highly potent and often deadly opioid, Hadland said.
“And increasingly there are secondhand exposures that are also rising,” Hadland, who participated in Emergent BioSolutions’ presentation, told the committee. “We’re seeing rising overdose deaths among toddlers who are coming across fentanyl in public settings or fentanyl that may be elsewhere in the home.”
Hadland said he tells parents to keep Narcan at their home in case of an emergency. He compared it to a fire extinguisher that families should have for safety reasons but hopefully will never have to use.
“Unfortunately for most young people, families and community members all across this country, current avenues of access are challenging,” Hadland said.
Dr. Bobby Mukkamala, of the American Medical Association, said Narcan should be as easy to obtain as Tylenol to treat a headache or a decongestant for a stuffy nose. Narcan should be just as common in public places as AED devices that are used to treat people suffering from heart attacks, Mukkamala said.
Jessica Hulsey, executive director of the Addiction Policy Forum, told the committee during a public comment section that Narcan needs to be priced affordably at no more than $20 per dose if it’s sold over the counter. “This is because Narcan is packaged as single doses and it can take multiple doses to reverse an overdose from highly potent fentanyl,” Hulsey said.
Narcan displaces opioids that bind to receptor sites in a person’s nervous system. By displacing and blocking opioids, the nasal spray prevents fatal overdoses by reversing respiratory depression, said Gay Owens, head of global medical affairs at Emergent BioSolutions.
However, Narcan must be administered as soon as an overdose is suspected, therefore the FDA’s Green stressed the importance of clear usage instructions for the nasal spray. The FDA’s advisors debated how to make the Narcan carton’s instructions as understandable as possible so that anyone can use the product easily in a life-threatening emergency.
In a study sponsored by Emergent BioSolutions, more than 90% of 71 participants understood over-the-counter label directions and used the Narcan device correctly during a simulated overdose emergency using mannequins. The participants included people with varying levels of literacy and both adults and adolescents.
The five-step instructions were spread throughout the side and back panels of the carton, which caused some participants to become confused, according to Millie Shah, a senior pharmacist at the FDA division that keeps track of medication administration mistakes. This confusion could result in delayed administration or errors in using the Narcan device correctly when time is of the essence, according to Shah.
These instances occurred despite the fact that the participants were allowed as much time as needed to familiarize themselves with the Narcan instructions, which may not be the case in a real-world overdose emergency, according to Shah.
“Therefore, the data collected does not capture this highest-risk-use scenario,” said Shah.
The FDA has recommended that Emergent BioSolutions sequentially sequence all five instructions and position them on the back panel of the carton and in the device blister pack. The company presented a mockup at the advisory meeting, but the FDA said it has not evaluated it yet.