Introduction
The papaya mealybug, P.marginatus Williams and Granara de Willink is a small, yellowish insect pest attacking papaya leaves and fruits and it belongs to the Family Pseudococcidae under the Order Hemiptera and Sub-order Homoptera. Its body is generally covered with thick waxy secretion which serves as meal for mainly small and big black ants, It is termed as mealybug because of its symbiotic association with ants. The ants help in the dispersal and transportation of this mealybug. According to Williams and Willink(1992), P.marginatus was first collected in Mexico during 1955. This species was described in1992 in the Neotropical Region occupying Belize, Costa Rica, Guatemala and Mexico. Walker et al. (2003) stated that P.marginatus was recorded from the 14 Caribbean countries.
Invasion of papaya mealybug in Asia during May,2008 was first reported by Muniappan et al.(2008) from Java, Indonesia and Tamil Nadu in India. In May, 2009 IPM (Integrated Pest Management) and CRSP(Collaborative Research Support Program) Scientists found Papaya Mealybug (PMB) at Joydebpur, Bangladesh. The damaging nature of PMB was observed in the BCSIR Vegetable Research Field during 2009.
P.marginatus is a noxious insect pest attacking papaya and other agricultural plants of economic importance. The abundance of this mealybug was observed during the months of March to August 2010 and 2011. During study period, five different kinds of insecticide plant materials viz., tobacco medicine (100%), tobacco leaf extract (20%), Mehgoni seed oil (5%), Neem seed oil (5%) and castor seed oil (5%). Mealybug infestation started from the past few decades, Among these biopesticides tested, tobacco medicine proved satisfactory in the suppression of this mealybug attack. Chemical control of P.marginatus is discouraged due to its some disadvantages polluting our environment. So, the role of biopesticide in the suppression of P.marginatus has been evaluated.
IDENTIFICATION
The papaya mealybug , Paracoccus marginatus is a small hemipteran insect belonging to the family Pseudococcidae . The first specimen of this devastating mealybug was collected in Mexico during 1955. It was described in 1992 in the Neotropical Region occupying Belize, Costa Rica , Guatemala , and Mexico.(Williams and Granara de Willink 1992 ).
Williams and De Boer (1973-1974) worked on the taxonomy of some Newzealand Pseudococcidae (Homoptera : Coccoidea ). The taxonomy of 10 spp. of Pseudococcidae from Newzealand was discussed . Two new genera (Paraferrisia and sarococcus ) and one new species (Trionymus wisei ) were described . Eight new species i.e; (Chorizococcus arecae , Dysmicoccus ambiuus , D. formicicola , Paracoccus morrisoni, Ripersiella rumicis , Trionymus sexaspinus , Sarococcus fagi , and Paraferrisia podocorpi ) were redescribed of which 7 were illustrated. The revision necessitates new combinations of names and new synonymy . Pseudococcus fargilis
Brain , a cosmopolitan species of economic importance, is made a synonym of P. calceolariae (Maskell) .
De Lotto (1975) narrated new species of Paracoccus Ezzat and McConell , 1956 ( Homoptera : Coccoidea ; Pseudococcidae ) from South Africa . Paracoccus claudus , P. larinus ; P.latebrosus and P. perperus were described . A key for the separation of the 7 spp. of the genus Paracoccus so far known from South Africa and South West Africa was also included in the check list ( inclusion of P.muraltiae , P. burnerae and P. mutabilis ).
Walker et al. (2003) stated that Paracoccus marginatus was recorded in the following 14 Caribbean countries i; e; St Martin , Guadeloupe, St Barthelemy , Antigua , Bahamas , British Virgin Island , Cuba , Dominican Republic , Haiti , Puerto Rico , Montserrat , Nevis , St Kitts and the U.S.Virgin Islands since 1994.
Muniappan et al.(2008) First Reported the papaya mealybug Paracoccus marginatus in Indonesia(Java) and India(Tamil Nadu). He also worked on the incidence and damage potential of this noxious pest .
According to Muniappan (2008) invasion of papaya mealybug in Asia during May 2008 , a team of scientists from the Integrated Pest Management Collaborative Research Support Program(IPM CRSP) supported by USAID found PMB (Papaya Mealybug) at the Bogor Botanical Gardens in Java Indonesia Subsequent surveys revealed that it was spread to Bali and Sulawesi Islands.
In July 2008, IPM CRSP scientists visiting coimbatore, India found PMB(Papaya Mealybug) infesting papaya in the orchard at Tamil Nadu Agricultural University.It has been since spread to three adjacent districts.In September 2008, IPM CRSP scientists helped to identify PMB(Papaya Mealybug) in Sri Lanka during November 2008.
Mapompeth (2009) reported Paracoccus marginatus in the northern part of Thailand. In May 2009, IPM CRSP scientists found PMB(Papaya Mealybug) at Joydebpur , Bangladesh during August 2009. The attack of this mealybugs was also confirmed in Maldives.
Indra et al. (2008) opined Paracoccus marginatus as an invasive mealybug attacking different crops of economic importance. The most recent report of its presence is from Malaysia in 2009. In Sri Lanka , papaya mealybug has been spread mainly to Gampaha, Colombo, Kurunagela and Kalutera districts. Higher infestation was observed in Gampaha and Colombo.
In a recent visit to Lahore (Mahmood and Solangi in March 15, 2011) observed the dispersal of this mealybug attacking unripe fruits and leaves of papaya in Pakistan.
BIOLOGY AND REPRODUCTION
Williams and Willink(2003) conducted research work on the effect of temperature on the life history of the mealybug Paracoccus marginatus and they also reported that Paracoccus marginatus was capable of developing and completing its life cycle at the temperature regimes i.e. 18,20,25, and 30±1ºC.
According to Walker et al.(2003) Papaya mealybug infestations are typically observed as clusters of cotton-like masses on the above ground portions of plants . The adult female is yellow and covered with a white waxy coating . Adult females are approximately 2.2 mm. long (1/16 inch) and 1.4 mm. wide .A series of short waxy caudal filaments less than 1/4th the length of the body exist around the margin . Adult males are approximately 1.0 mm long, with an elongate-oval body which is widest at the thorax(0.3 mm). Adult males have ten-segmented antennae and well-developed wings . Details on the biology and life cycle of the papaya mealybug are lacking . In general,mealybugs have piercing-sucking mouth-parts and feed by inserting their stylets into plant tissue and
sucking out sap . Mealybugs are most active in warm and dry weather. Females have no wings and move by crawling short distances or by being blown in air currents. Females usually lay 100 to 600 eggs in an ovisac , although some species of mealybugs give birth to youngs. Egglaying usually accomplished in the period of one to two weeks. Egg is hatched in about 10 days and the nearly emerged nymphs or crawlers begin to actively search for feeding sites. Female crawlers have four instars , with a generation having approximately one months duration for completing its life cycle depending on the other prevailing temperature and environmental conditions .
Tanwar et al.(2010) worked on the incidence and damaging valuve of papaya mealybug and its management strategies. Papaya mealybug are most active in warm and temperature weather. An individual female usually deposits 100 to 600 eggs. Eggs are greenish yellow and are laid in an ovisac which is about three to four times the body length and entirely covered with white wax. Eggs generally hatch at nearly 10 days and nymph or crawlers pass their times in search of feeding locations. Males have longer developmental time (27-30 days) than females (24-26 days) at 25±1ºC 65±2% RH and 12:12(L:D) photoperiod. Aitken 1894,Thangamalar et al.(2007) described papaya mealybug , P. marginatus , as an invasive pest from central American countries. This mealybug has caused havoc in agricultural and horticultural crops in India ever since its first report from coimbatore during 2007. The authors have reported that the adult females of P. marginatus laid eggs (approximately about 150 to 200 eggs) inside the egg-sacs. Eggs are pink coloured, grain like measuring 0.120 cm in diameter.
Indra et al.(2008) carried out research work on P. marginatus . The female mealybugs usually lay upto 600 eggs enclosed in an ovisac. P. marginatus was observed to complete the life cycle on papaya (Carica papaya L.) in 26 days and the life cycle was found to vary from 15 days to 32 days depending on the host plant species. It has the ability to develop, survive, and reproduce successfully between 18 to 30ºC which suggests that it has the ability to develop and establish in areas within this temperature range.
HOST PLANTS
Miller & Gray (2002) worked on the incidence and developmental stage of P. marginatus in different host plants in U.S.A . The genus Paracoccus includes some 79 species of varied distribution from the “Austro-Oriental, Ethiopian ,Madagasian, Nearctic, Neotropical, Newzealand , Pacific, Palaearetic and oriental regions” (Ben Dov 1994).Although most assigned species have not been recognized as major economic pest, there are two notable exception. P.marginatus is a polyphagous insect , it has recorded on about 55 host plants in more than 25 genera. Economically important host plants of this severe pest are shown in Table 01.
Table 01. Host plants and References of Paracoccus marginatus.
| Acacia sp. | Miller et al. (1999) |
| Acalypha sp. | Miller et al.(1999) |
| Acalypha wilkesiana Muell.-Arg. | Hamon (personal communication) |
| Ambrosia cumanensis auct.non Kunth | Williams and Granara de Willink (1992) |
| Annona.squamosa L. | Miller et al.(1999) |
| Bauhinia sp. | Hamon (personal communication) |
| Carica papaya L. | Hamon (personal communication) |
| Carica sp. | Williams and Granara de Willink (1992) |
| Cestrum nocturnum L. | Hamon (personal communication) |
| Citrux x paradisi Macfad.(pro sp.) | Hamon (personal communication) |
| Clerodendrum panicuatum L. | Hamon (personal communication) |
| Coccoloba sp. | Miller et al. (1999) |
| Fistulosa sp. | Hamon (personal communication) |
| Guazuma ulmifolia Lam. | Miller et al. (1999) |
| Hamelia patens jacq. | Hamon (personal communication) |
| Hamelia sp. | Hamon (personal communication) |
| Hibiscus sp. | Hamon (personal communication) |
| Hibiscus rosa-sinensis L. | Hamon (personal communication) |
| Ipomoea carnea jacq. | Hamon (personal communication) |
| Ipomoea sp. | Miller et al. (1999) |
| Jatropha integerrima jacq. | Hamon (personal communication) |
| Jatropha sp. | Hamon (personal communication) |
| Malvaviscus penduliflorus DC. | Hamon (personal communication) |
| Manihot chlorosticta Standl.& Goldman | Williams and Granara de Willink (1992), Miller et al. (1999) |
| Manihot esculenta Crantz | Williams and Granara de Willink (1992), Miller et al. (1999) |
| Mimosa pigra L. | Williams and Granara de Willink (1992) |
| Parthenium hysterophorus L. | Williams and Granara de Willink (1992), Miller et al. (1999) |
| Persea americana P. Mill. | Miller et al. (1999) |
| Plumeria rubra L. | Hamon (personal communication) |
| Plumeria sp. | Hamon (personal communication) |
| Rhaphiolepis umbellate (Thunb.) Makino | Hamon (personal communication) |
| Sida sp. | Williams and Granara de Willink (1992), Miller et al. (1999) |
| Solanum melongena L. | Miller et al. (1999) |
| Uniola paniculata L. | Hamon (personal communication)s |
| Zea mays L. | Miller et al. (1999) |
Indra et al. (2008) reported that P. marginatus is a polyphagous pest and it has been recorded on about 55 host plants in more than 25 genera. Economically important host plants of this mealybug include papaya, hibiscus, pomegranate, avocado, citrus, cotton, tomato, eggplant, peppers, beans, peas, sweet potato, mango, cherry etc. In Sri Lanka P. marginatus has been reported in about 30 families of host plants. However, papaya (Carica papaya L.) had been recorded as the most preferred host plant while Manioc (M. utilissima) and temple trees (Plumeria acuminata) as the next preferred ones.
In Bangladesh there are many host plants of P. marginatus At present a considerable number of host plants of papaya mealybug have been encountered attacking different vegetable, fruit and ornamental categories belonging to different families of Plant kingdom. The name of these host plants are listed below:
Table 02: List of recorded hosts of papaya mealybug, P. marginatus in Bangladesh (Plant Names of Bangladesh ; Native and Scientific by A.H.HUQ, June 1986)
Native names | Scientific names |
| Am | Mangifera indica L. |
| Anaras | Ananas sativus schult. |
| Arhar, Arhar-Dal | Cajanus cajan (L.) Millsp. |
| Babla | Acacia nilotica (L.) |
| Begun | Solanum melongena wall. (Solana.) |
| Bhutta | Zea mays L. (Gramineae) |
| Dalim | Punica granatum L. (Punicaceae) |
| Belati-gab | Disopyros philippensis (Desr.) |
| Desi-gab | Disopyros peregrina (Gaertn.) |
| Dhanchi (Din) | Pologonum fagopyrum L. |
| Dheras | Abelmoschus esculentus (L.) Moen |
| Hital | Phoenix paludosa Roxb. (Palmae) |
| Ipil-ipil | Leucaena latisiliqua (L.) Gills (Legum.) |
| Jaba | Hibiscus rosa-sinensis L. (Malvaceae) |
| Kanthal | Artocarpus heterophyllus Lamk. |
| Karpas, Karpastula | Gossypium harbaceum L. (Malvaceae) |
| Lebu | Citrus aurantifolia (Christ.) |
| Pepe | Carica papaya L. (Caricaceae) |
| Peyara | Psidium guajava (L.) Bat. (Myrtaceae) |
| Seuli | Nyctanthes arbortristis L. (Oleaceae) |
| Shimul | Bombax ceiba L. (Bombacaceae) |
| Sisu | Dalbergia sissoo Roxb. (Leguminosae) |
| Til | Sesamum indicum L. (Pedaliaceae) |
| Tomato | Lycopersicon lycopersicum (L.) Karst. |
| Tulshi | Ocimum americanum L. (Labiatae) |
| Misti alu | Ipomoea batatas Lamk. (Convolvu.) |
DAMAGE
Walker et al. (2003) conducted on the different ecological aspects P. marginatus. The papaya mealybug feeds on the sap of plants by inserting its stylets or beaks into the epidermis of the leaves, as well as into the un-ripe fruits and stems. In doing so, it injects a toxic or harmful substance into the leaves. The result is chlorosis, plant stunting, leaf deformation, early leaf and fruit drop, a heavy buildup of honeydew, and death of host plants. Heavy infestations are capable of rendering fruit inedible due to the aggregation of thick white waxy appearance, papaya mealybug has only been recorded feeding on the areas of the host plant above ground parts including leaves and fruits of different host plants.
P. marginatus is a polyphagous pest, with hosts recorded from 22 plant families. Infestations of PMB (Papya Mealybug) accur along the veins of older leaves and on all parts of young leaves and fruits. Honeydew secrated by this mealybug results in the development of sooty mold (fungus) that covers leaves, fruits and stems, impeding photosynthesis and gaseous exchange and papaya trees usually die within a few months after infestation.
MANAGEMENT
A. Chemical Control
Walker et al. (2003) described a number of chemical weapons to control mealybug, although none are currently registered specifically for control of papaya mealybug. Active ingredients in registered pesticide formulations include acephate, carboryl, chlorpyrifos, diazinon, dimethoate, malathion, and white mineral oils. Typically, twice the normal dose is applied when treating for mealybugs because mealybugs are protected by thick waxy, cottony sacs, and often are concealed inside damaged leaves and buds.
Thus, chemical controls are only partially effective and require multiple applications.
Furthermore, problems with insecticide resistance and non-target effects on natural enemies make chemical control a less desirable control option to combat the papaya mealybug.
B. Biological control
Walker et al. (2003) reported that natural enemies of the papaya mealybug include the commercially available mealybug destroyer (Cryptolaemus montrouzieri), lady beetles, lacewings, and hover flies, all which are generalist, predators that have a potential impact on Agricultural Research Service (ARS) initiated a classical biological control program for the papaya mealybug. Four genera of encyrtid endoparasitoid wasps specific to mealybug were collected in Mexico by USDA and ARS researchers and Mexican cooperators as potential biological control agents. Acerophagus papayae (Noyes and Schauff), Anagyrus loecki (Noyes and Menezes). Anagyrus californicus Compere and pseudaphycus sp (USDA, 1999, 2000, Meyerdirk and Kauffman 2001). A fifth collected species was later reared and identified as Pseudleptomastix mexicana (Noyes and Schauff 2003).
Accroding to Muniappan (2008), classical biological control approach of P. marginatus is an exotic or introduced pest in Asia and it is suitable for the classical biological control approach of releasing species-specific parasitoids. This approach has been successfully implemented against PMB (papaya mealybug) in several countries in the Caribbean, some islands in the pacific and in the states of Florida and Hawaii in the United States.
In May 2009, three parasitoids (Anagyrus loceki, Acerophagous papayae and Pseudleptommastrix mexicana (Hymenoptera:Encyrtidae) obtained from the USDA APHIS parasitoid rearing facility at Puerto Rico were released to Sri Lanka.
By August 2009, there were reports of controlling PMB up to 95 to 100% in some parts of the country and both India and Indonesia are planning to introduce these parasitoids from Sri Lanka. One or more of these parasitoids have fortuitously established in Maldives.
Muniappan et al. (2008) reported Classical Biological Control of the papaya mealybug, P. marginatus (Hemiptera: Pseudococcidae in the Republic of Palau, South America. The papaya mealybug P. marginatus is a pest in Central America and the Caribbean, was noted to have been established on Palau in March 2003 and was causing serious damage to papaya, plumeria, hibiscus and other plants. The parasitoids Anagyrus loecki Noyes and Schauff (Hymenoptera: Encyrtidae) totaling 24, 586 were imported from Puerto Rico and field released in Palau from August 2003 to June 2004. Their attempts in the suppression of this havoc creating mealybug has been proved successful depending on the prevailing environmental and ecological conditions of the above mentioned countries.
MATERIALS AND METHODS
The present research work was confined to the study of mealybug , P.marginatus Williams and Granara de Willink. It belongs to the order Hemiptera Sub-order Homoptera under the Family Pseudococcidae. This is usually called as papaya mealybug. It is usually quite small, yellowish in appearance and inconspicuous and is found on the leaves, twig, stems, and fruits of the host plants. Females are elongate oval with distinct segmentation and covered with waxy secretion which may be extended into lateral or terminal filaments. P.marginatus was collected from papaya leaves and unripe fruit of plants in the BCSIR Vegetables Research Field from April to September 2011 for experimental purposes.
STUDY PROCEDURE
Study period of this research work was initiated in March, 2010 and ended in August, 2011. Collection sites were BCSIR Vegetable Research Field and adjacent areas of DhakaCity. During survey on the incidence of this mealybug the parameters considered were: temperature relative humidity photoperiod rainfall etc. P.marginatuswas collected with the help of fine and soft camel brush and forceps.
SAMPLING METHOD
Different nymphal stages, male and female adults of P.marginatus were collected randomly from papaya plants at fortnightly intervals. The collected specimens were kept in different glass vials and small petridishes (6 cm diameter).
PRESERVATION
The mealybug P.marginatus and some beneficial hymenopteran insect parasites belonging to the Family Encyrtidae, Braconidae and other insects associated with this mealybug were preserved in 80% alcohol with a drop of glycerine.
MOUNTING
The insects were mounted temporarily on slides in lectophenol and permanent mounts were also made for study purposes. The specimens were dechitinized in hot KOH solution before processing. These were dehydrated in desired grades of ethyl alcohol and mounted in canada balsam along with xylene solvent. The mounted specimens were labeled properly for future study purpose or references.
OBSERVATION
The specimens or newly prepared slides were examined under a Binocular Dissecting Microscope and a Compound Microscope.
MEASURMENT AND DRAWING
A simple millimeter scale was used in Binocular Dissecting Microscope for taking necessary measurements of egg, nymphal stages and adults of P.marginatus. Stage micrometer also used for calibration. Measurements of minute parts were taken with the help of ocular micrometer fitted to the compound microscope.
PHOTOGRAPHY
For the purpose of accurate, detailed and distinct description microphotographs of different nymphal stages and adult male and female P.marginatus were drawn at the Zoology Section, BCSIR Laboratories , Dhaka with the aid of Digital Camera (Canon Power Shot A 470, 7.1 pixel)
To depict a clear picture of P.marginatus infesting unripe fruits and leaves of papaya plants some photographs in natural habitat were also taken.
IDENTIFICATION
Taxonomic identification of the mealybug, Paracoccus marginatus was performed earlier by Dr. Nurul Alam , C.S.O.; BARI, Joydebpur, Gazipur from the International Institute of Entomology London, U.K. Our collected specimen of mealybug was compared with his identified specimen from 11E and confirmed our collected specimens as P. marginatus Granara de Willink (Hemiptera : Pseudococcidae). We also confirmed this specimen as P. marginatus dissolving in ethanol when it turned bluish-black in colour.
TAXONOMIC FEATURE
The external morphological characters of different nymphal stages as well as adult P.marginatus were studied briefly and these are presented in Taxonomy and Biology Chapter.
SEASONAL ABUNDANCE
For conducting the experiments on the fluctuation and seasonal abundance of P.marginatus , some papaya plants of the experimental field was selected randomly and then its occurrence i.e. total numbers of mealybug in a single papaya fruit/leaf of each plant was marked, counted and recorded fortnightly. In this manner, the occurrence of mealybugs during the months from March to August, 2010 and 2011 were taken and calculated carefully and then abundance of mealybugs was estimated as total numbers of mealybugs observed and recorded for study purpose. The highest and lowest occurrence of mealybugs were also taken into consideration. Collected samples of papaya fruits and leaves having mealybug infestations were taken in the laboratory room and reared. The samples were observed carefully for insect predator or parasitoid emergence, if occurs, any.
PREPARATION OF BIOPESTICIDES FOR FIELD APPLICATION
This experiment was conducted from July to September, 2010 and April to June, 2011. During study period, five kinds of indigenous plant materials or biopesticides use collected/prepared to find out the effect of biopesticide in the suppression of P.marginatus .A control experiment was also made for comparison. The biopesticides applied were:
1) Tobaco Medicine(100%)
2) Tobaco Leaf Extract(20%)
3) Mehgoni Seed Oil(5%)
4) Castor Seed Oil(5%)
5) Neem Seed Oil(5%)
For preparation of tobacco medicine, 200 gm of dried tobacco leaf were immersed into 1 litre of water for 24 hours. Then this water extract of tobacco is mixed 2% calcium carbonate. Now, this extract is termed as tobacco medicine (100%), Mehgoni, Neem and castor seed oils are purchased from New Market, Dhaka and applied in papaya plants after making desired percentage with plain water.
INSECT AND PARASITOID COLLECTION
Many insects including black ants were found associated with P. marginatus. Some encyrtid and braconid insect parasitoids belonging to the Order Hymenoptera and other insects were collected observed with this mealybug. These were collected and preserved for future study.
BIOLOGY OF Paracoccus marginatus WITH
TAXONOMIC CHARACTERS
RESULT AND DISCUSSION
Taxonomic Status of P. marginatus is given below:
Kingdom: Animalia
Phylum: Arthropoda
Class: Insecta
Order: Hemiptera
Suh-order: Homoptera
Family: Geometroidea
Subfamily: Aphidinae
Genus: Paracoccus (Sulzer,1776)
Specific name: marginatus-Williams &Granara de Willink, 1992
Scientific name: Paracoccus marginatus
Williams & Granara de Willink,1992
The papaya mealybug, P marginatus Williams and Granara de Willink (Hemiptera: Pseudococcidae) is a small polyphagons sucking insect. This noxious insect pest passes through egg and three nymphal stages to become adult. Its embryonic, development is termed as pauro metabolic metamorphosis or simple metamorphosis. In case of P.marginatus generally four and three nymphal instars take place in case of male and female mealybug respectively.Biology morphometrics and some taxonomic details of P. marginatus are described briefly as follows:
Egg
P. marginatus adults are most active in warm, dry and humid weather. Females have no wings and move by crawling short distances or by being blown in air currents. Females usually lay 100 to 600 eggs. Eggs are greenish yellow in colour and are laid in an ovisac that is 3-4 times the body length and entirely covered with white waxy substance. The ovisac is developed ventrally on the adult female. Egglaying is continued over a period of 1-2 weeks.
The adult female of P marginatus laid about 150 to 200 eggs inside the ovisacs. Eggs are pink coloured, grain like measuring 0.120 cm in diameter.
Thangamalar et al. (2010) observed that the fecundity of P. marginatus, at 20 and 25o C, they described that eggs hatched in about 3-4 days and 10 days at the temperature gradients respectively. After hatching, the nymphs or crawlers begin to actively search for feeding sites for their survival and dispersal.
Nymphal stages
There are three nymphal stages and no pupal stage in the life cycle of a wingless female and eggs are laid in a small, white ovisac of woolly wax. Whereas males pass through four instars to become adult. Males have longer development time (27-30 days) than females (24-26 days) at 25 ± 1o C, 65 ± 2% RH and 12 : 12(light : dark) photoperiod.
First-Instar nymph (Gender not determined)
In the present study, the length of first instar nymph was 0.42 ± 0.074 mm and 0.27± 0.024 mm in width. This instar appears yellowish in colour.
Dorsum with 9(7-10) pairs of cerarii ; cerarii indefinite, cerarii with 2 conical setae. Cerarius 12 absent. Cerarius in anal lobe without auxiliary setae, 2 conical setae, 1 trilocular pore; Dorsal body setae more slender than cerarian setae. Multi-locular pores absent; triloculars pores scattered over surface, forming 2 longitudinal lines on each side of abdomen, excluding cerariian setae. Discoidal pores absent. Oral-rim tubular ducts and oral-collar tubular duets are absent. Longest sub-medial seta on 7th segment 5(4-8) m long; without sub-medial setae on 8th segment. Slide-mounted characters – Body 0.4 (0.3-0.6) mm long and 0.2 (0.2-0.3) mm wide. Similar taxonomic features were described by (Douglass et al. 2002).
Second-Instar Female
In the present study, the length of second instar nymph was 0.6 ± 0.054 mm and 0.4 ± 0.089 mm in width. Body colour appears yellow in field condition.
Dorsum with average 6 (4-11) pairs of cerarii ; cerarii indefinite, cerarii with 2 conical setae. Cerarius 12 absent. Anal lobe cerarius with 1 auxiliary setae, 2 conical seate, 2 (2-3) trilocular pores, sometimes with 1 discoidal pore. Dorsal body setae more slender than cerarian setae. Multilocular pore absent; trilocular pores scattered over surface, most abundant near setae ; discoidal pores rare, about ½ diameter of trilocular pore. Oral-rim tubular duets absent. Longest sub-medial seta on 6th segment about 6(5-6) m long; sub-medial setae located on the 8th segment is devoid of setae. . Slide-mounted characters-Body 0.7(0.5-0.8) mm long and 0.4 (0.3-0.5) mm wide. Taxonomic characters in cerarius setae resmbles male. Similar taxonomic features were described by (Douglass et al. 2002).
Third-Instar Feamle
In the present study, the length of third instar nymph was 0.89 ± 0.111 mm and 0.51 ± 0.02 mm in width. Body looks like yellow in colour during field observation. Dorsum with 6(1-10) paris of cerarii ; cerarii indefinite, when present, with 2-conical setae and 1 trilocular pore between conical setae. Cerorius 12 absent. Anal lobe cerarius with 1(1-2) auxiliary setae, 2 conical setae, 5 (4-7) trilocular pores, 0(0-1) discoidal pores. Dorsal body setae more slender than cerarian setae. Multilocular pores absent; trilocular pores scattered over surface, most abundant near setae; discoidal pores rare, about ½ diameter of trilocular pore. Oral-rim tubular duct rarely present near position of cerarius 8(of 10 specimens examined, 4 had 1 oral rim or large oral-collar on at least one side of body). Oral-colar tubular ducts absent. Longest sub-medial seta on the 7th segment is 7(5-10) m long; 1(0-2) sub-medial setae located on 8th segment. Slide-mounted characters: Body 1.1 (0.7-1.8) mm long, 0.7(0.3-1.1) mm wide. Similar taxonomic features were described by (Douglass et al. 2002)
Adult Female
The live adult covered with powdery, white wax and without any longitudinal depressions. Short waxy filaments develop around the body margin including short caudal filaments. The body contents are yellow when alive but turn black in less than one day after death, even when preserved in ethyl alcohol. In the present study, the length of adult female was 2.08 ± 0.354 mm and 1 ± 0.063 mm in width. Body looks like yellow in colour during field observation.
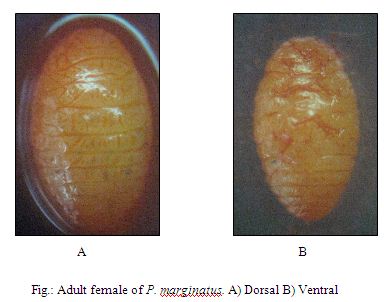
Dorsum with 16(14-17) pairs of cerarii; cerarii 1, 2, 4, 5, 7 and 9 with 2 conical. Setae ; cerarii 3, 6 and 16 with 3(2-3) conical setae; cerarii 8, 11 and 17 with 2(0-2); cerarii 10 and 14 with 1(0-2) conical setae; cerarii 12, 13 and 15 with 2(0-3) conical setae. Cerarius 12 without auxiliary setae. with 2(0-3) conical setae, 5(0-8) trilocular pores, 1(0-3) discoidal pores. Anal lobe cerarius with 1(1-3) auxiliary setae, 2 conical setae, 13(10-18) trilocular pores, 2(0-3) discoidal pores. Dorsal body setae more slender than cerarian setae. Multilocular pores absent; trilocular pores scattered over surface, most abundant near setae; discoidal pores rare, about ½ diameter of trilocular pore, oral-rim tubular ducts usually restricted to marginal areas associated with cerarii, 1 specimen examined with 1 mediolateral duct on segment 1 and 1 in medial area of mesothorax of 21 specimens examined. In slide mounted adult females from the oriental region, this is the only species of Paracoccus that totally lacks, oral-rim ducts in the sub-median or median areas of the dorsum. Slide-mounted characters: Body 2.2 (1.5-2.7) mm in length and 1.4(0.9-1.7) mm in width. Similar taxonomic features were described by (Douglass et al. 2002).
Second-Instar Male
In the present study, the length of second instar nymph was 0.6 ± 0.054 mm and 0.4 ± 0.089 mm in width. Body looks like yellow in colour during field observation.
Dorsum with 4 (2-5) pairs of cerarii; ceranii indefinite, when present, with 2 conical setae and 1 trilocular pore between conical setae. Cerarius 12 absent. Anal-lobe cerarius with 1(1-2) auxiliary setae, 2 concial setae, 2(2-3) trilocular pores, without discoidal pores. Dorsal body setae more slender than cerarian setae. With 1(0-2) multilocular pores in medial areas of thorax and head, present on 6 out of 10 specimens examined; trilocular pores scattered over surface, most abundant near setae; discoidal pores rare, about ½ diameter of trilocular pore. Oral-rim tubular ducts absent. Oral-collar tubular ducts abundant over surface of 1 size longest sub-medial seta present on segment 6th segment end 6(6-8) m long. No submedial setae on the 8th segment. Slide-mounted characters- Body 0.6(0.5-1.0) mm long and 0.3 (0.2-0.6) mm wide. Similar taxonomic features were described by (Douglass et al. 2002).
Third-Instar Male (prepupa)
In the present study, the length of third instar nymph was 0.23 ± 1.05 mm and 0.59 ± 0.156 mm in width. Body looks like yellow in colour during field observation.
Dorsum without cerarii; posterolateral margins of segments 5 or 6, 7 and 8 each with 2 setae conspicuously longer than remaining setae on abdominal segments. Multilocular pores in medial areas of head, forming row on prothorax and metathorax, usually without pores on mesothorax, occasionally with 1 or 2 medially, in rows on most abdominal segments, fewer in medial area, absent from 8th and 9th segments, trilocular pores absent; discoidal pores rare. Oral-rim tubular ducts absent. Oral-collar tubular ducts present around body margin, medial and submedial ducts sometimes present on prothorax and metathorax and with 1 or 2 abdominal segments. Longest submedial seta on the 7th segment 18(15-20) m long; without submedial setae on the 8th segment. Slide-mounted characters: Body 0.9 (0.8-1.1) mm long, 0.4 (0.3-0.4) mm wide. Similar taxonomic features were described by (Douglass et al. 2002).
Notes
The above description is based on 20 observations having 5 replication.The prepupa can be distinguished from all other instars by having multilocular pores, oral-collar tubular ducts antennae without definite segmentation, tibia and tarsus fused, no labium, no aedeagus and no definite constriction for the head.
Fourth-Instar Male (Pupa)
In the present study, the length of fourth instar nymph was 0.98 ± 0.075 mm and 0.49 ± 0.02 mm in width.Body colour appears pink but occasionally yellowish. Dorsum without cerarii; posterolateral margins of segments 3, 4 or 5 to segment 8 each with 2 setae conspicuously longer than remaining setae on the abdominal segments. Multilocular pores absent from head, forming conspicuous row on prothorax, mediolateral clusters on metathorax, without pores on mesothorax. In mediolateral clusters on each side of abdominal segments of 1-6 or 7 trilocular pores absent; discoidal pores associated with multiloculars and oral collars. Oral-rim tubular ducts absent. Oral-collar tubular ducts present near body margin of prothorax and the abdominal segments 1 or 2 to 7 or 8 forming clusters ducts. Longest submedial seta on is located the 7th segment and 20(16-28) m in length. Slide mounted characters: Body 1.0 (0.9-1.0) mm in length and, 0.3(0.3-0.4) mm in width. Similar taxonomic features were described by (Douglass et al. 2002).
Adult Male
In the present study, the length of adult male was 1.5 mm and 0.5 mm in width.Body colour appears pink, but occasionally yellowish.
Adult males have 10-segmented antennae, a distinct aedeagus, lateral pore clusters, a heavily sclerotized thorax and head, and well-developed wings. Slide-mounted characters: Body enlongate oval, 1.0(0.9-1.1) mm long; greatest width at thorax and 0.3(0.2-0.3) mm. Similar taxonomic features were described by (Afifi,S. 1968, Douglass et al. 2002).
Ecology
The optimum temperature for the development of paracoccus marginatus is between 20 to 25o C and the minimum temperature is 21o C. Developmental times for male and female nymphs and adult males are similar at 25 and 30o C
HOST PLANTS OF P. marginatus
RESULT AND DISCUSSION
Paracoccus marginatus is a small polyphagous sucking pest, and has been recorded as a devastating pest on about 55 host plants in more than 25 genera. Economically important host plants of it include papaya, hibiscus, avocado, citrus, cotton, tomato, eggplant, peppers, beans, peas, sweet, potato, mango, cherry, and pomegranate etc. It also attacks many tropical fruits, vegetables and ornamental plants.
In Sri Lanka, P. marginatus has been reported in about 30 families of different host plants. However, papaya (Carica papaya L.) plant is the most preferred host while Manioc (M. utilissima) and temple trees (plumeria acuminata)as the next preferred ones. (Wahundeniya et al. 2008)
Muniappan (2008) Tanwar et al. (2010) heavy attack of papaya mealybug in India has been noticed on a large number of cultivated crops and weed hosts belonging to different Families of plant kingdom. The following table provides the list of so far recorded host of P. marginatus from different countries of the world including Bangladesh.
FLUCTUATION AND SEASONAL ABUNDANCE OF Paracoccus marginatus
RESULTS AND DISCUSSION
In the present study, the seasonal abundance of the insect pest, Paracoccus marginatus associated with unripe papaya fruit, in BCSIR experimental field of Dhaka city was investigated. The insects of P. marginatus were also collected from different localities of Dhaka city and BCSIR experimental Field. Relevant studies were carried out in the research laboratory of Zoology Section, BCSIR Laboratories, Dhaka.
Effect of temperature on the life history of the mealybug P. marginatus was investigated in the laboratory. P. marginatus developed and completed its life cycle at 18, 20, 25 and 30 ± 10 C. Females passed through 3 instars whereas males had four instars. Males have longer developmental time (27-30 days) than those of females (24-26 days) at 25 ± 10 C, 65 ± 2% RH and 12 : 12 (L:D) Photoperiod.(Tanwar et al. 2010).
Temperature showed significant influence on the reproduction and multiplication of this mealybug. It also had pronounced effect on the seasonal abundance of the pest. The fluctuation and seasonal abundance of P. marginatus is presented.
According to the estimated minimum temperature thresholds for the adult males and females were 14.5 and 13.9oC respectively. For adult males, the estimated optimum and maximum temperature thresholds were 28.7 and 31.9oC and for adult females, they were 28.4 and 32.1oC, respectively. The ability of P. marginatus to develop, survive and reproduce successfully between 18 and 30oC suggests that it has the capability to develop and establish in areas within this temperature range.
With rapid development, high survival rate, and enormous reproductive capacity, P. marginatus population could potentially reach a high level. Thus, temperature and humid it (65-70% R.H.) fovoured the seasonal abundance and reproductive potential of the P. marginatus.
Papaya mealybugs are most active in warm and dry weather. The wax, which sticks to each ovisac and nymphs, also facilitate passive dispersal by equipment, animals or human beings. The female mealybug is not active and unable to fly. In fact, human beings greatly facilitate in the transport of these mealybugs. Long-distance movement is aided through transport of infested planting material and fresh fruits and vegetables from one end of a farm to the other or even across the country.
In May to July and October to December each year papaya mealybug infestation appears on above ground parts on leaves, stem and fruits as clusters of cotton-like masses. Papaya mealybug has only been recorded feeding on areas of the host plant that are above ground, namely the leaves and fruit.
The insect sucks the sap by inserting it stylets or beak into the epidermis of the leaf, fruit, stem and bud. While feeding, it injects a toxic substance into the leaves resulting in chlorosis, plant stunting, leaf deformation or crinkling, early leaf and fruit drop, a heavy deposition of honeydew and death of plants.
DAMAGE POTENTIAL OF P. marginatus
RESULTS AND DISCUSSION
During present study,P. marginatus was found to severely attack immature and mature papaya fruits and tender underneath leaves of this plant. Due to serious attack the pericarp of papaya fruit was damaged and growth of fruits were cuased to some extent. Ultimately the papaya fruits became unsuitable for human consumption. It was a striking feature to state that the infestation of this mealybug was initiated in the older or ripe leaves of the plants from lower portions. Then the population of P. marginatus was increased and the active nymphs and adults moved towards immature papaya fruits of the plant. The mealybugs were found gregariously sucking on the precarp of the unripe papaya fruits. The gregarious attacking nature are given bellow in Figs. A, B, C .

Infestations of PMB occur along the veins of older leaves and on all parts of young leaves and fruits. Honeydew secreted by this mealybug results in the development of black Sooty mold that covers leaves, fruits and stems impeding photosynthesis and gaseous exchange. As a consequence, papaya trees die within a few months after infestation. (Muniappan 2008).
The peak infestation of P. marginatus was during from the month of April- August, 2011. The minimum infestation was noticed during September to October each year. Due to severe infestation, premature falling of leaves and immature papaya fruits occur.
Paracoccus marginatus is a small polyphagous sucking insect. It has been recorded from 22 plant families. Infestation of the mealybug appears as clusters of cotton-like masses on the above-ground portion of plants with long waxy filaments. Immature and adult stages of P. marginatus suck the sap of the plant and weaken it. The leaves become crinkled and wrinkled yellowish and wither. The honeydew secreted by the bug and associated black sooty mould formation hampers photosynthetic efficiency of the affected plants (Tanwar et al. 2010).
Healthy plants might be infested from mealybug infested plants as juvenile mealybugs crawl from an infested plant to another plant. Small ‘crawlers’ get readily dispersed by wind, rain, irrigation water, birds, ants, clothing, and vehicle etc. So, the population of P. marginatu gradually increases. In certain crops like cotton, stems which often carry mealybug infestation are stocked in the farm for propagation or other purposes. These stocks, near the newly planted crop act as reservoirs of papaya mealybug ( Tanwar et al. 2010).
The papaya mealybug feeds on the sap of plants by inserting its stylets into the epidermis of the leaf, as well as into the fruit and stem. In doing so, it injects a toxic substance into the leaves. The result is chlorosis, plant stunting, leaf deformation, early dropping of leaves and fruits a heavy accumulation of honeydew, and death of the host plants. Heavy infestations are capable of rendering fruit inedible due to the deposition of thick white wax by this mealybug has only been recorded feeding on different locations of the host plant that are above ground, namely the leaves and fruits.
Black and Red ants, attracted by the honeydew, have been seen carrying mealybug from plant to plant. So, ants act as a mode of dispersal of this noxious insect pest.
Mealybugs are known to offer ants with their sugary secretion (honeydew) and in return ants help in spreading the mealybugs and provide protection from predator ladybird beetles, parasitoids and other natural enemies, Ants also keep the papaya mealybug colony clean from detritus that accumulate in the secreted honeydew, which may be harmful to the mealybug colony. Some small and big ants i.e; Oecophylla smaragdina,Componotus compressus, have been found attending papaya mealybug, feeding on honeydew on jatropha, papaya and other plants. So, it is apparent that the ants act as a symbiotic agent for betterment, survival and wide dispersal of P. marginatus.
CONTROL STRATEGIES OF P.marginatus
The papaya mealybug colonize at the lower side of the papaya leaves along the veins and later move to unripe fruits rendering them unmarketable and inedible for human consumption. The loss appeared to be in the range 70-95 percent in my present study. Generally young plants are killed due to heavy infestation and colony formation of this mealybug.
P. marginatus is a polyphagous pest attacking several agricultural, horticultural crops and weeds of economic value.
P. marginatus Williams and Granara de Willink (Hemiptera : Pseudococcidae) a pest in Central America and the Caribbean, was noted to have been established on Palau in March 2003 and was causing serious damage to papaya and other plants. Chemical control is only partially effective and requires repeated and multiple applications. The chemical insecticides are recommended as the last weapon to the suppression of mealybug such as profenophos 50EC (2 ml/litre), chlorpyriphos 20 EC (2 ml/litre), buprofezin 25 EC (2ml/litre), dimethoate 30 EC (2 ml/litre), thiomethoxam 25 WG (0.6 g/litre), imidaeloprid 17.8 SL(0.6 ml/litre).
Active ingredients in registered pesticide formulations include acephate, carbaryl, chlorpyrifos , diazinon, dimethoate, malathion and white mineral oils. Typically, twice the normal dose is applied when treating for mealybugs because mealybugs are protected by thick waxy, cottony sacs, and often are concealed inside damaged leaves and buds. Thus, chemical controls are only partially effective and require multiple appilactions.
Furthermore, problems with insecticide resistance and non-target effects on natural enemies make chemical control a less desirable control option to combat the papaya mealybug.Natural enemies of the papaya mealybug include the commercially available mealybug coleopteran predator, Cryptolaemus montrouzieri, lady beetles, lacewings, and hover flies, all which are generalist predators that have a potential impact in the suppressions of mealybug population. In addition to predators, several parasitoids may attack papaya mealybug.
In 1999, the USDA Animal and Plant Health Inspection Service (APHIS)and USDA Agricultural Research Service (ARS) initiated a classical biological control programme for the papaya mealybug. Four genera of encyrtid endoparasitoid wasps specific to mealybug were collected in Mexico cooperators as potential biological control agents viz.
1. Acerophagus papayae (Noyes and Schauff)
2. Anagyrus loecki (Noyes and Menezes)
3. Anagyrus californicus compare, and
4. Pseudaphycus sp.(USDA 1999, 2000; Meyerdirk and Kauffman 2001).A fifth collected species was later reared and identified as Pseudleptomastix mexicana (Noyes and Schauff 2003).

All four species were screened in USDA/ARS quarantine facilities in Newark. Delaware and environmental assessments were completed (USDA-APHIS 1999,2000,2002). Specimens were then shipped to Puerto Rico where they were cultured and massreared for experimental release in Puerto Rico and the Dominican Republic . The first releases of these four parasitoids were made in Florida in October, 2000. APHIS has conducted the release of the four genera of parasitoid wasps has brought a 99.7% reduction in the density of mealybug population at research sites in the Dominican Republic, and a 97% reduction at research sites in Puerto Rico, with parasitim levels between 35.5% and 58.3% (Kauffman et al. 2001, Meyerdirk and Kauffman 2001). All four species of parasitoids have been observed attacking second and third instars of P. marginatus. However, Acerophagus sp. emerged as the dominant parasitoid species in both Puerto Rico and the Dominican Republic (Meyerdirk and Kauffman 2001). The out-come of releases of the four parasitoids in Florida is yet to be determined as released in March 2003.
The papaya mealybug has been controlled successfully by the parasitoid Acerophagus papayae Noyes and Schauff (Hymenoptera: Encyrtidae) imported from Puerto Rico where A. papayae appeared to provide 99% effective control. Biological control, which is the most successful method of control of this pest has been successfully implemented in Florida, Caribbean Islands, countries in South America, Guam and Palau. Sri Lanka, too, is in the process of importing the parasitoid, A. papayae introduced in May 15, 2009.
There is a need to conserve the native predators of the pest. Australian ladybird beetle (Cryptolaemus montrouzieri) predates on mealybugs, eating 3,000-5,000 mealybugs in various life stages and is released @ 10 beetles per tree or @ 5,000 beetles/ha.
Exotic parasitoids/predators such as Anagyrus loecki, Acerophagous papayae and Pseudleptomastrix mexicana (Hymenoptera: Encyrtidae) were released in Sri Lanka in May 2009 (imported from Puerto Rico) and resulted in 95 to 100% control of the papaya mealybug in some parts of that country by August 2009.
There is a need to introduce such exotic parasitoids in India and Bangladesh to control P. marginatus and to maintain pollution free environment . Further, it is suggested to locate ant colonies and destroy them with drenching of chlorpyriphos 20 EC @ 2 ml/litre of water which may be a part in the suppression of this mealybug.
Biopesticide Control Approach of P. marginatus.
In this chapter effect of some biopesticides on the mortality of nymphal and adut of P. marginatus have been evaluated. The potential biopesticides which were selected were : tobacco leaf (with stalk) extract, Neem seed oil, Mehgoni seed oil and castor seed oil.
Regular monitoring of the crop for occurrence of mealybug infestation and its natural enemies were performed . Biopesticide application were started immediately after noticing mealybugs on some papaya plants in the field conditions.
Biopesticides or botanicals such as Neem seed oil (5%), Mehgoni seed oil (5%), Castor seed oil (5%), dried tobacco medicine (100%), dried tobacco leaf extract (20%), were sprayed to the affected leaves and immature and mature fruits of different papaya plants containg mealybugs. Four fruits were ripen and these fruits were earlier sprayed by MO(5%) during end of April, 2011. The fruits were not palatable because of severe attack of mealybug infestation. The nymphs and adults of P. marginatus sucked the pericarp by immature and mature fruits by inserting their stylets into them. So, distate occurred. On the other hand, the ripe papaya fruits from uninfested papaya plants were tasty. Observations were made after 24 hours to determine the effect of these Biopesticidies on the mortality of the mealybugs, if any.
A control experiment was maintained having mealybug intestation untreated. The mortality in different treatments were counted and recorded carefully for 3 consecutive days. The effect of different biopesticides on the mortality of P. marginatus is presented in Table 07.
CONCLUSION
P.marginatus is a widespread hemipteran mealybug infesting different vegetable and agricultural crops of economic values. Due to severe attack, immature and mature papaya fruits drop and the plants become stunted and die gradually. Besides, damaged fruits become inedible for human consumption.
P.marginatus attack becomes peak in July annually. The least infestation was recorded during September each year. Five biopesticides namely, tobacco medicine, tobacco leaf extract, Mehgoni seed oil, Neem seed oil and castor oil were sprayed to the affected papaya plants. Tobacco medicine (100%) exerted the best result relating to control.
In recent years, P.marginatus has been recognised as a cosmopolitan and devastating pest causing enormous damage to the vegetables, fruits, other agricultural and ornamental plants. It has now become an alarming pest in many countries of the world including Bangladesh, Pakistan and India. So, the control measure of this papaya mealybug needs special attention to the Entomlogists’ in the present situation. If we become successful in controlling this havoc-creating mealybug through biopesticide, then it may produce significant impact in the socio-economic development of Bangladesh keeping sound environment.