Alzheimer’s disease research on marmosets is an intriguing method to acquiring a better knowledge of this neurodegenerative condition. Marmosets are small primates that share some genetic and physiological characteristics with humans, making them a potentially excellent model for Alzheimer’s disease study.
Neuroscientists developed the first non-human primate model of familial Alzheimer’s disease in marmosets to speed up medication discovery and lay the groundwork for future translational studies.
University of Pittsburgh School of Medicine neuroscientists created the first non-human primate model of hereditary Alzheimer’s disease in marmoset monkeys to reimagine existing preclinical trials for Alzheimer’s disease, outlining their approach in Alzheimer’s & Dementia: Translational Research & Clinical Interventions.
Researchers are now aiming to characterize and validate genetic, biochemical, functional, and cognitive characteristics of aging and Alzheimer’s disease in marmosets with mutations in the same gene related to early-onset dementia in people. Scientists want to speed up the drug discovery pipeline and lay the groundwork for future translational studies while overcoming the constraints of existing preclinical models.
We are ambitious about finding a cure for Alzheimer’s disease. We are establishing a process for rigorous, minimally invasive standardized testing of the marmoset model of Alzheimer’s disease and openly sharing data.
Afonso Silva
“We are ambitious about finding a cure for Alzheimer’s disease,” said senior author Afonso Silva, Ph.D., professor of neurobiology at Pitt. “We are establishing a process for rigorous, minimally invasive standardized testing of the marmoset model of Alzheimer’s disease and openly sharing data.”
Marmoset families are more closely related to the genetically heterogeneous human population than an inbred rodent colony. Because marmosets have shorter life spans than other non-human primates, researchers can examine their aging in depth in a relatively short amount of time.
If allowed to mature naturally, marmosets will acquire toxic amyloid beta and tau clumps in the brain, indicating Alzheimer’s-like disease. Researchers used the Crispr/Cas9 gene editing technique to generate a succession of mutations in the PSEN1 gene to develop marmosets with an inheritable vulnerability to Alzheimer’s disease. In humans, these same mutations cause early-onset Alzheimer’s disease.
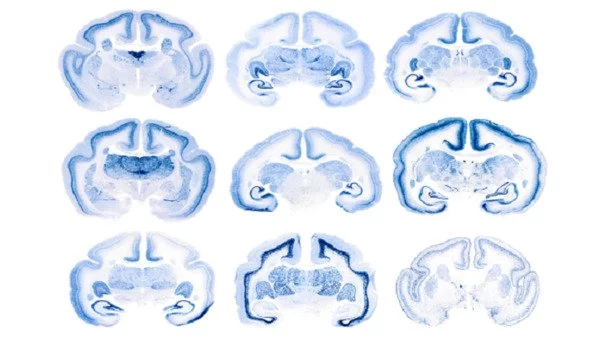
Presenilin-1, the protein encoded by PSEN1, plays a key role in generating amyloid tangles, and, just like human patients, marmosets with a mutation in the PSEN1 gene start developing Alzheimer’s-like pathologies during adolescence.
The team is developing the model by treating marmosets as if they were human patients, using a bench-to-bedside method. Researchers are using a battery of non-invasive tests to describe and validate the new model, including behavioral investigations, longitudinal monitoring of blood biomarkers, and regular PET scans to assess brain function and pathological changes in brain tissue. The experiments are intended to map and compare the aging trajectory of healthy controls and animals genetically susceptible to early-onset Alzheimer’s disease, as well as to correlate progressive changes in amyloid and tau levels to changes in cognition.
Fig: Researchers to study Alzheimer’s disease in marmosets
Marmosets are used in scientific research because they are genetically closer to humans than many other non-human primates. They have a relatively short lifespan, which allows researchers to study the progression of Alzheimer’s disease over a shorter time frame compared to using other animal models.
Researchers also intend to investigate additional characteristics associated with illness development, such as brain-blood barrier permeability, vascular stiffness, and metabolism, as well as examine changes in gene expression profiles over time by sampling skin cells on a regular basis.
“We need new models to understand the underlying biological processes behind normal and pathological aging,” said Stacey Rizzo, Ph.D., associate professor of neurobiology and deputy director of preclinical research at Pitt’s Aging Institute. “Tracking the animals from birth in a well-controlled way would allow us to hypothesize on how molecular and genetic changes translate to pathophysiological consequences in the brain and devise ways to stop them from getting to the point of no return.”