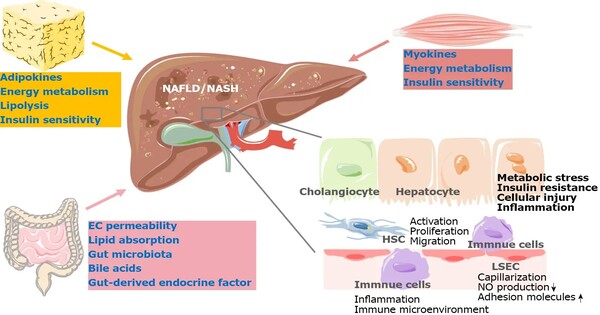
Metabolic dysfunction-associated steatotic liver disease (MASLD), formerly known as “non-alcoholic fatty liver disease,” affects approximately 25% of the global population. The severe type, metabolic dysfunction-associated steatohepatitis (MASH), can cause liver fibrosis and even liver failure. With only one approved treatment now available, identifying solutions for MASLD and MASH is critical.
Obesity, a poor diet, and a lack of exercise are all risk factors for MASLD and MASH. These disorders cause fat to accumulate in the liver, resulting in inflammation and scarring. Over time, this can proceed to fibrosis and cirrhosis, causing significant liver damage. Despite their widespread prevalence, MASLD and MASH patients have few treatment options.
The findings suggest that inhibiting ACMSD could be a promising new treatment for MASLD and MASH. Increasing NAD+ production in the liver may protect against the serious damage caused by these disorders, lowering the risk of progression to cirrhosis.
Johan Auwerx
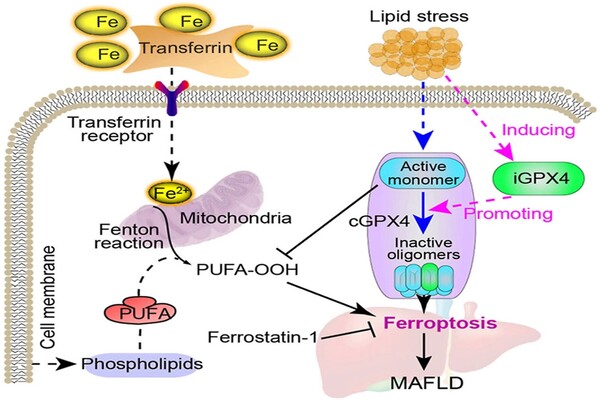
Another problem is the reduced levels of a molecule called NAD+ (nicotinamide adenine dinucleotide), which plays a key role in many cellular processes, including energy production, DNA repair, and inflammation control. In MASLD/MASH, NAD+ levels drop, and this contributes to liver damage and disease progression. Restoring NAD+ levels could potentially stop or even reverse this damage — the question is, how?
A team of scientists led by Johan Auwerx at EPFL has now shown that inhibiting an enzyme called ACMSD could be the answer. ACMSD (α-amino-β-carboxymuconate-ε-semialdehyde decarboxylase) is mainly found in the liver and kidneys and is involved in breaking down the amino acid tryptophan and limiting the production of NAD+. By blocking ACMSD, the researchers found they could increase NAD+ levels in the liver, which in turn reduced inflammation, DNA damage, and fibrosis in mouse models of MASLD/MASH.
The researchers used a variety of models, including mouse liver cells and human liver organoids (lab-grown mini-liver). They also fed mice a Western-style diet heavy in fat to simulate the conditions that induce MASLD/MASH in humans. After the disease had progressed in the mice, they administered an ACMSD inhibitor named TLC-065 and assessed its effects on liver function and NAD+ levels in the mouse liver, as well as on human liver organoids.
The results were promising. Inhibiting ACMSD considerably increased NAD+ levels, notably in the liver, where ACMSD plays an important role in energy metabolism and DNA protection. This increase in NAD+ lowered inflammation, reversed fibrosis, and repaired DNA damage in the treated mice’s livers. Meanwhile, they discovered that suppressing ACMSD in human liver organoids lowers signs of DNA damage.
The findings suggest that inhibiting ACMSD could be a promising new treatment for MASLD and MASH. Increasing NAD+ production in the liver may protect against the serious damage caused by these disorders, lowering the risk of progression to cirrhosis. This strategy also emphasizes the importance of metabolic pathways in liver disease and introduces ACMSD as a novel target for therapeutic development.