Despite the fact that many forms of autism spectrum disorder (ASD) are thought to have genetic causes, the cellular and molecular functions of the identified genes are unknown. Scientists at Austria’s Institute of Science and Technology (IST) investigated a high-risk gene and discovered its critical role during a critical stage of brain development.
Autism spectrum disorder affects approximately three million people in the European Union alone (ASD). Some people are only mildly affected and are able to live independently. Others are severely disabled. The difficulties with social interaction and communication, as well as repetitive-stereotypic behaviors, are shared by the various forms. ASD is linked to mutations in a few hundred genes. Cullin 3 is one of them, and it is a high-risk gene: A mutation in this gene almost always results in a disorder. But how does this gene influence the brain?
To find out more, Jasmin Morandell and Lena Schwarz, Ph.D. students in Professor Gaia Novarino’s lab, compared mice with partially deactivated Cullin 3 genes to their healthy siblings. Their findings were recently published in the journal Nature Communications.
Although many forms of autism spectrum disorder (ASD) are thought to have genetic causes, the cellular and molecular functions of the identified genes remain unclear. Scientists studied a high-risk gene and discovered its important role during a critical phase of brain development.
The team wanted to see if the modified mice mimicked some of the characteristics of patients with this type of autism and could thus be used as model organisms in a series of behavioral and motoric tests. A mouse could freely explore three adjacent chambers of a box connected by small doors in one of these tests, known as the three-chamber sociability test. The scientists then placed two other mice in the outer boxes, one of which was already familiar to the studied mouse and the other with which it had never previously interacted.
“Healthy mice usually prefer the new over the already familiar mouse,” explains Jasmin Morandell, the study’s co-first author. The mouse with the mutated Cullin 3 gene, on the other hand, showed no signs of recognition. Furthermore, the mice exhibited deficits in motor coordination as well as other ASD-related cognitive impairments. The team was then able to get to the bottom of the mechanisms that cause these changes with the help of this mouse model.
A Dangerous Accumulation of Proteins
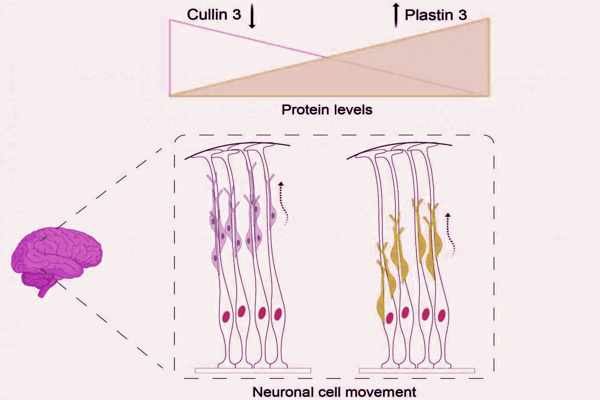
The researchers noticed a very subtle but consistent change in the position of some brain cells while studying the mouse brain. These neurons, also known as nerve cells, are derived from a specific region of the brain. From there, they progress to the cortex’s uppermost layers until they reach their destination. It is a very sensitive process in which even minor changes in the rate at which they travel can alter the structure of the cortex. The scientists were able to track the movement of the migrating neurons by labeling them. “We could see migration deficits — the neurons are stranded in the lower cortex layers,” Lena Schwarz, the study’s other co-first author, explains. But why are the cells not moving as they should?
The answer lies in the critical role Cullin 3 plays in protein degradation. When the time comes, the gene Cullin 3 marks them for degradation, a process that must be tightly regulated to prevent protein accumulation. Morandell and Schwarz examined the protein composition of the mouse brain to determine which proteins are misregulated when Cullin 3 is deficient. “We were looking at proteins that accumulated in the mutant brain when we came across Plastin 3. Then, in the hallway, Gaia discovered a poster describing the work of IST Austria’s Schur group, and we became very excited “Jasmin Morandell says.
“They had been working on Plastin 3 as a regulator of cell motility independently and had similar results to ours. That’s when we started collaborating “Prof. Gaia Novarino recalls.
Plastin 3, a previously unknown protein in the context of neuronal cell migration, was discovered to play an important role in this process. “When the Cullin 3 gene is silenced, the Plastin 3 protein accumulates, causing cells to move more slowly and over shorter distances. This is exactly what we observed in the cortex of Cullin 3 mutant mice “Lena Schwarz, a Ph.D. student, agrees.
A Risky Pathway?
All of this is happening at a very early stage of brain development, around halfway through pregnancy, long before anyone notices a difference in the fetus. “Determining these critical windows during brain development could be extremely important for fine-tuning the treatment of patients with specific forms of ASD,” says Novarino, who is dedicated to improving ASD diagnosis and treatment options. “Following up on the Plastin 3 research could pave the way for some therapeutics. Inhibiting the accumulation of this protein may eventually relieve some of the symptoms experienced by patients “According to Schwarz.
“We now know that a faulty Cullin 3 causes an increase in Plastin 3. This close relationship suggests that Plastin 3 protein levels may be an important factor in the regulation of cell-intrinsic movements “Jasmin Morandell says She recently graduated and hopes to apply her knowledge of brain development to research Huntington’s disease. Lena Schwarz will then look at other high-risk ASD genes to see if other proteins in the degradation pathway are linked to ASD. The Novarino group collaborated with the Danzl and Schur groups, as well as a colleague from the University of Rome, on the current study.
“Finishing this extensive study in around two and a half years despite the pandemic was only possible with the support from our neighbors at IST Austria,” Novarino praises the multidisciplinarity at the Institute.