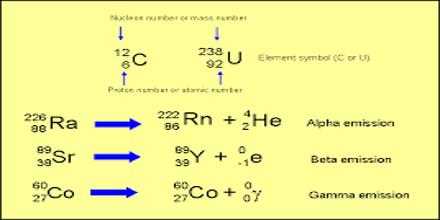
A nuclear equation is a symbolic representation of a nuclear reaction using nuclide symbols. For example, the nuclide symbol for uranium-238. The total charge is conserved during a nuclear reaction. This means that the sum of the subscripts for the products must equal the sum of the subscripts for the reactants. The rules for balancing nuclear equations are different from those we have been using for balancing ordinary chemical equations.
Presentation on Nuclear Equations