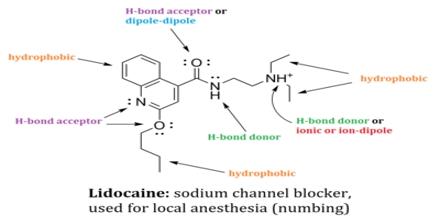
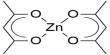
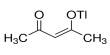
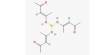
Non-covalent interactions differs from a covalent bond in that no involve the giving of electrons, but alternatively involves more spread variations of electromagnetic relationships between molecules or inside a molecule. There are four main kinds of non-covalent interactions in biological systems: hydrogen bonds, ionic bonds, lorrie der Waals relationships, and hydrophobic bonds. The bond energies for these interactions cover anything from about 1 to 5 kcal/mol.
Non-covalent Interactions