INTRODUCTION:
Lucilia cuprina (Wiedemann) is commonly known as The Australian Sheep Blowfly. It is a fly of medical and veterinary importance, not only as an ectoparasite, but also because it causes myiasis in humans and other mammals, particularly sheep.
Over 240 thousands metric tons of marine fishes are sun dried in Bangladesh for both local consumption and export purposes. Nazirertek, Naniachatta, and off shore Sonadia island are three major fish drying yards of Cox’s Bazar among many other fish drying stations along the coastal belts of Bangladesh. A number of Diptera are known to damage sea fish during sun-drying. Among those, L. cuprina is regarded as the most destructive pest for fish drying industries in Bangladesh. They seriously affect the fish industries of the off-shore islands in the Bay of Bengal (Huda 2000). Over 25% of marine fishes is lost due to fly infestation besides there is quality deterioration of the product during the process of sun drying (Doe et al. 1977). The fish driers usually dip the fish in insecticide prior to sun-drying to avoid infestation and thus, posing health hazards, environmental degradation and reducing acceptability of dried fish in both national and international markets. The feasibility study of using the Sterile Insect Technique (SIT) is being implemented in Sonadia island in order to control or suppress L. cuprina as an effective, environment friendly technique. The off-shore Sonadia island of the Bay of Bengal around 4 km away from the Cox’s Bazar sea shore, has been chosen as an isolated area with relatively less possibility of reinvasion of the pest that is necessary for successful application of SIT control strategy.
The biological aspects of some blowfly species were studied by Sukontason et al. (2004) in Aldrichina grahami and Chrysomya pacifica; by Begum et al. (2007) in C. rufifacis; by walker (1994) in C. bezziana; by Prins (1979) in C. megacephala and C. bezziana; by Gare et al. (2005) and Esser (1991) in C. megacephala and by Etienne (1969) in Dacus cucurbitae.
Objectives:In the present study on biological aspects of L. cuprina would be useful for implementing pre and post release investigations necessary for evaluating efficacy of SIT control strategy being implemented at Sonadia marine fish drying yards against the fly pest .
MATERIAL AND METHODS:
The biology of L. cuprina was studied under the laboratory conditions (25±5ºC, R H 70±10% and 12 L:12 D) over a period of one year (July 2006 to June 2007). The work was carried out at the Blowfly Laboratory of Radiation Entomology and Acarology Division, Institute of Food and Radiation Biology, Atomic Energy Research Establishment, Savar, Dhaka.
A stock rearing with suitable rearing materials of L. cuprina was maintained in the laboratory. Adult stock was kept in rectangular cages (12″× 8″× 8″) made up of wood/steel frame and covered with nylon mosquito net. The adult flies were supplied with sugar and water soaked cotton wool as food. Small pieces of fresh bovine liver were kept in a petri dish inside the stock rearing cage on which the females laid eggs.
To observe the life cycle stages, a small mass of freshly laid eggs was gently scraped from the liver and put on a fish (Talapia sp.) in a small bowl. The fish was used as the food of the larvae hatched. The small larval rearing bowls were usually placed in a large plastic bowl containing a relatively thick (2-3 inches) layer of sawdust. The large bowl was finally covered with a piece of cloth in order to prevent possible invasion of the same or different fly species. The newly emerged larvae started feeding on the supplied fish as soon as they came out from egg shell, and the feeding continued up to the 3rd instar larvae. The late 3rd instar larvae usually crawled over the wall of small bowl and dropped on to the sawdust of the larger plastic bowl prior to pupation. The pupae were separated from the sawdust by hand. The clean pupae were kept in a petri dish and then were placed in the adult cages covered with mosquito net for adult emergence.
The different morphological features and changes of various life stages were observed under a light microscope and microphotographed by using a Nikon microscope having photographic arrangements. The eggs, larvae, post-fedding larvae and pupae were preserved in 70% alcohol in airtight screw-capped vials, and the length and breadth of them were measured with the help of an ocular micrometer.
The longevity of adult males and females was observed on two different food media (liver+sugar+water and sugar+water) for both mated and unmated conditions. The longevity of mated males and females was studied under two conditions : one with liver and the other without liver. Ten pairs of males and females were introduced separately into single pair breeding cages (8″x 6″x 6″). On the other hand, for the longevity of unmated male and female, supplied with liver and without liver, ten pairs of males and females were introduced separately into breeding cages (8″x 6″x 6″). The newly emerged adults were separated with the help of a test tube and a light microscope.
A pair of newly emerged adults of opposite sexes was introduced into breeding cages (8″x 6″x 6″) and was supplied with liver, sugar and water. The number of eggs laid was counted daily throughout the entire oviposition period.
The hatching percentage was calculated after placing the eggs in a linear fashion on the pieces of wet red cloth on Petri dish for facilitating counting of hatched and unhatched eggs. The cloth was generally kept wet continuously by placing a small lump of water soaked cotton in contact with the cloth. The total number of hatched and unhatched eggs were recorded under a light microscope after twenty four hours of their placement. The total oviposition period was divided into two parts as early oviposition period and late oviposition period. The number of eggs laid by a female fly and the hatch percentage were recorded for both early and late oviposition periods.
RESULTS AND DISCUSSION:
The life cycle of L. cuprina exhibits complete metamorphosis showing egg, larva, pupa and adult stages.
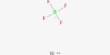
Egg (Fig. 1, 2): The egg of L. cuprina was white, cylindrical. The eggs were almost rounded at both ends and the anterior end was more tapered than posterior end. The egg shell or chorion was thick and hard. The apparent white eggs attained a deeper cream colouration prior to hatching. Under light microscope, segmental dark bands and black pointed mouth hook of the developing larva were visible through the transparency of the chorion. The first instar larva hatched through a longitudinal split made at the anterior end of the egg shell. A hatching line enclosing a median strip occurred on the dorsal side of the egg, along the entire length and the presence of a bifurcation at the micropyle is closely similar to the findings of O’Flynn and Moorehouse (1980) in the eggs of L. cuprina.
Figs. 2-3: 2 Magnified view of egges of L. cuprina showing micropyle and hatching line
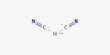
at. 3a. Anterior end of third instar larva of L. cuprina showing mouth hooks and anterior spiracle. 3b. Last abdominal segment of the same showing paried spiracles.
The mean length and breadth of the eggs were 1.06±0.05 and 0.30±0.02 mm, respectively (Table 1). The incubation period of L. cuprina was 10.00±1.23 hours (Table 2).
Larva (Fig. 1): The larvae of L. cuprina were acephalous and apodous type which had three larval instars. The mouth of the larva was situated at the anterior end of the body containing a pair of hooks used for feeding.
The first instar larva (Fig. 1): It was elongated and white to deep creamy in colour. The early larvae were relatively more transparent than late stage of development. The body was composed of 12 segments. The 1st instar larva was relatively broader at the posterior end than the anterior end. The mouth hooks were situated middorsally. The spines were thornlike, black in color and remain backwardly directed. The prothoracic spiracle was a small, simple opening. The paired posterior spiracle possed a single opening but without any peritreme. The duration of 1st instar larva was 12.80±1.48 hours (Table 2). The mean length and breadth of it was 1.65±0.15 and 0.36±0.04 mm respectively (Table 1).
The second instar larva (Fig. 1): The 2nd instar larva was deep creamy to pale yellowish in colour. Spines in bands were present in each segment. Each of the pair of posterior spiracles remained surrounded by a heavily sclerotized peritreme, brown in colour which was incomplete or nearly so dorsally and ventrally. The mean duration of the second instar larvae was 23.00±2.92 hours (Table 2). The mean length and breadth for the second instar larvae were 3.06±0.31 and 0.63±0.14 mm respectively (Table 1).
The third instar larva (Fig. 1): They were deep creamy to pale brownish in colour. The food materials in the digestive tract were apparent through the body cuticle. The relatively heavy bands of dark, robust and thornlike spines were relatively more prominent than those of the 2nd instar larva. Each of the posterior spiracles was surrounded by a heavily sclerotized peritreme, possessing three slit-like spiracular openings. The spiracles were blackish brown in color. The mean duration of 3rd instar larvae was 36.40±4.39 hours (Table 2). The mean length and breadth of 3rd instar larvae were 10.10±0.57 and 1.45±0.28 mm respectively (Table 1).
The results of the present investigation on the larval morphology of L. cuprina are largely supported by the findings of Queiroz and Carhalvo (1987).
The total larval period: The duration from the newly hatched larva to the dropping out of 3rd instar larva was 72.20±8.26 hours.
Post-feeding larval stage (Fig. 1): At the end of the third instar, the larvae became sluggish and stopped feeding, entered into a wandering phase and generally left the feeding site. It responded negatively to light and usually aggregated on sawdust under the feeding bowl. The post feeding larva underwent a dramatic alteration in shape, became wider and shorter with gradual contraction, hardening, shortening and darkening of cuticle till formation of the pupa. The colour of this stage was brillient creamy white and no food materials were apparent in the body. The mean duration of post feeding larval stage was 22.00±3.16 hours (Table 2). The mean length and breadth of the post-feeding larval stage were 9.70±0.59 and 1.75±0.26 mm, respectively (Table 1).
Pupa (Fig. 1): The squeezed post-feeding larvae underwent a process of pupariation during which the cuticle of the larvae became heavily sclerotized. The type of the pupae were coarctate adecticous. The posterior end of the puparium was relatively round while the anterior end was a little more pointed. Some cuticular characteristics of the third instar larva especially the bands of spines, black sclerotized mouth-hook, anterior and posterior spiracles etc. were retained on the puparium. The pupa changed colouration from creamy white to light brown to red brown and finally to almost blackish brown during the completion of sclerotization in few hours to a day. They were apparently more blackish during adult emergence. The mean duration of pupal stage was 5.00±0.71days (Table 2). The mean length and breadth of the pupae were 6.60±0.39 and 1.85±0.24 mm respectively (Table 1).
Adult (Fig. 1): When the adult was ready to emerge from the puparium it shed the pupal cuticle. The newly emerged flies were at first very soft, grayish black in colour with ptilinum and unexpanded wings. The ptilinum was inverted within some hours, the cuticle hardened and the wings attained normal shape and colouration. The adults subsequently attained metallic green colouration. The males usually emerged earliar than the females. The males were relatively smaller in size than the adult females. The mean length and breadth (at the thoracic region) of the adult males were 6.80±0.42 and 1.95±0.37 mm, respectively and of the females were 8.25±0.49 and 2.55±0.37 mm, respectively (Table 1).
Life cycle (egg to adult emergence) (Fig. 1): The mean time required for L. cuprina from egg to adult emergence was 9.34±0.96 days (Table 2).
Adult longevity: The longevity of male was usually shorter than that of female. The liver fed adults survived longer than that of liver unfed ones. The longevity of mated and unmated male reared on liver, sugar and water were 27.10±5.04 days and 19.10±3.60 days, and of mated and unmated female reared on the same food were 34.20±3.85 days and 25.50±4.58 days, respectively (Table 3). On the other hand, the longevity of mated and unmated male reared on sugar and water were 18.30±3.53 and 17.30±4.16 days, and of mated and unmated female reared on the same food were 20.40±4.14 and 19.70±3.83 days, respectively (Table 3).
Fecundity : The mean fecundity was 906.75±466.03. The mean fecundity during early and late oviposition period were 528.80±303.41 and 378.85±203±54 respectively (Table 4). The result shows that the females during their early reproductive period were more fecund than those of late reproductive period. The fecundity of L. cuprina was significantly greater in early oviposition phase (P< 0.05). Hatching percentage: The hatching percentage of the larva was 72.48±27.17 and 65.96±27.44 respectively (Table 4). The eggs laid during early reproductive period were more viable than those laid by females during late reproductive phase.
Oviposition behaviour: The flies generally deposited the eggs in the cavities of liver supplied as media for egg laying. The egg masses were brilliant white remained firmly attached to each other and substratum as well. The eggs were glued to each other along their long axes. The eggs were characteristically laid parallel to each other, giving the appearance of a shingled roof. The individual eggs were difficult to separate from an egg mass without the use of water or chemical solvents. In a large population the same oviposition sites were utilized by several females and they laid their eggs in the form of a composite egg mass, which is supported by the findings of Browne (1958) in case of L. cuprina; and Byrd and Butler (1996) and Begum et al. (2007) in C. rufifacies. This behaviour might help in protecting the eggs from desiccation and increase the chance of establishment of a population in a new area. The ovipositing female usually laid eggs in two or more smaller egg masses while it was disturbed during oviposition. The eggs were laid readily by a female when it took nourishment from the liver. It was also found that the female laid maximum eggs on fresh liver than the older one . The present observation is similar to those of Kamal (1958) who opined that fresh beef liver was more suitable medium for oviposition of calliphorids than old liver and Browne (1958) who reported that the female of L. cuprina had a preference for ovipositing in cavities in the fleece, especially those near the moist cotton wool plugs.
CONCLUSION:
The knowledge gained on the life cycle and other biological aspects of the experimental fly would be helpful for the development of a suitable SIT control strategy against L. cuprina. These information may be utilized for other control measures including application of indigenous natural enemies.
ACKNOWLEDGEMENTS:
The authors sincerely appreciate the financial assistance of the R and D project of Bangladesh Atomic Energy Commission entitled “Suppression\ eradication of blowfly infesting sun dried fish in the off shore islands and coastal areas of Bangladesh by the application of Sterile Insect Technique (SIT). The authors are grateful to the authority of the Radiation Entomology and Acarology division of the Institute of Food and Radiation Biology, Savar, Dhaka for providing equipments and materials necessary for carrying out of the research work.
LITERATURE CITED:
BEGUM, S., NASIM, M. and MAJUMDER, M.Z.R. 2007. Some biological aspects of the hairy
maggotfly, C. rufifacies (Macquart, 1842) (Diptera: Calliphoridae). Bangladesh J.
Zool. 35(2):367-380.
BROWNE, L.B. 1958. The choice of commercial oviposition sites by the Australian sheep
blowfly Lucilia cuprina (Wiedemann) (Calliphoridea: iptera). Aust. J. Zool. 6:241-
247.
BYRD, J.H. and BUTLER, J.F. 1996. Effect of temperature on Chrysomya rufifacies (Diptera: Calliphoridae) development. J. Med. Entomol. 34:353-358.
DOE, P.E., AHMED, M., MUSLEMUDDIN, M. and SCHITHANANTHAN, K. 1977. A polythene tent drier for improved sun drying of fish. Food Technol. Aust. 29(11):437-441.
ETIENNE, J. 1969. Lutte control les mouches des fruits (control of fruit flies). Rapp. Inst. Rech. Agron. Trop. Reunion.1969. pp. 251-259.
ESSER, J.R. 1991. Biology of Chrysomya megacephala (Diptera: Calliphoridae) and reduction of losses caused to the salted-dried fish industry in South-.East Asia. Bull. Entomol. Res. 81(1):33-41.
GABRE, R.M.; ADHAM, F.K. and CHI, H. 2005. Life table of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae). Acta. Oecologica. 27:179-183.
HUDA, S.M.S. 2000. Longevity and mating competitiveness of irradiated and
unirradiated wild type female of Lucilia cuprina (Wied.) (Diptera:Calliphoridae.
Banggladesh J. Life Sci. 12(1):99-102.
KAMAL, A.S. 1958. Comparative study of thirteen species of Sarcosaprophagous Calliphoridae and Sarcophagidae (Diptera). Bionomics. Ann. ent. Soc. Am. 51:261-271.
O’FLYNN, M.A. and MOOREHOUSE, D.E. 1980. Identification of early immature
stages of some common Queensland carrionflies. J. Aust. Ento. Soc. 19:53-61.
PRINS, A. J. 1979. Discovery of the oriental latrine fly, Chrysomya megacephala (Fabricius) along the South-western coast of South Africa. Ann. S. Afr. Mus. 78:39-47.
QUEIROZ, S.M. and CARVALHO, C.J.B. 1987. Chave pictorial e descricoes de larvas de 3rd inster de Diptera (Calliphoridae, Muscidae e Fannidae) em vazadouros e residuos solidos domesticos em curitiba. PR. Ann. Soc. Entomol. Bras. 16:165-188.
SUKONTASON, K., SUKONTASON, K. L., PIANGHAI, S., CHOOCHOTE, W., BOONCHU, N., CHAIWONG, T. and KURAHASHI, H. 2004. Fine structure of the eggs of blowflies Aldrichina grahami and Chrysomya pacifica (Diptera: Calliphoridae). Biol.Res. 37:483-487.
WALKER, A. 1994. The arthropods of human and domestic animals. A guide to preliminary identification (2nd ed). Chapman and Hall London. pp. 85-129.