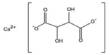
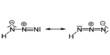
Bismuth is a chemical element with symbol Bi and atomic number 83. It is mainly used as an alloying component in fusible alloys. Elemental bismuth may occur naturally, although its sulfide and oxide form important commercial ores. The free element is 86% as dense as lead. It is a brittle metal with a silvery white color when freshly produced, but surface oxidation can give it a pink tinge.
Bismuth compounds account for about half the production of bismuth. They are used in cosmetics, pigments, and a few pharmaceuticals, notably bismuth subsalicylate, used to treat diarrhea. Bismuth’s unusual propensity to expand upon freezing is responsible for some of its uses, such as in casting of printing type. Bismuth has unusually low toxicity for a heavy metal.
Physical properties
- Phase (at STP): solid
- Melting point: 544.7 K (271.5 °C, 520.7 °F)
- Boiling point: 1837 K (1564 °C, 2847 °F)
- Density (near r.t.): 9.78 g/cm3; when liquid (at m.p.) 05 g/cm3
- Heat of fusion: 11.30 kJ/mol
- Heat of vaporization: 179 kJ/mol
- Molar heat capacity: 25.52 J/(mol·K).

Chemical Properties
- Density: 9.80 g.cm-3 at 20°C
- Melting point: 271 °C
- Boiling point: 1420 °C
- Vanderwaals radius: 0.152 nm
- Ionic radius: 0.074 nm (+5) ; 0,120 nm (+3)
- Isotopes: 14
Occurrence
In the Earth’s crust, bismuth is about twice as abundant as gold. The most important ores of bismuth are bismuthinite and bismite. Native bismuth is known from Australia, Bolivia, and China.
According to the United States Geological Survey, the world mining production of bismuth in 2014 was 13,600 tonnes, with the major contributions from China (7,600 tonnes), Vietnam (4,950 tonnes) and Mexico (948 tonnes). The refinery production in 2010 was 16,000 tonnes, of which China produced 13,000, Mexico 850 and Belgium 800 tonnes. The difference reflects bismuth’s status as a byproduct of extraction of other metals such as lead, copper, tin, molybdenum, and tungsten. World bismuth production from refineries is a more complete and reliable statistic.
Applications
Bismuth has few commercial applications, and those applications that use it generally require small quantities relative to other raw materials. In the United States, for example, 884 tonnes of bismuth were consumed in 2010, of which 63% went into chemicals (including pharmaceuticals, pigments, and cosmetics); 26% into metallurgical additives for casting and galvanizing; 7% into bismuth alloys, solders and ammunition; and 4% into research and other uses.
Some manufacturers use bismuth as a substitute in equipment for potable water systems such as valves to meet “lead-free” mandates in the U.S. (began in 2014). This is a fairly large application since it covers all residential and commercial building construction.
Information Source: