Biography of Michael Faraday
Michael Faraday – British scientist.
Name: Michael Faraday
Date of Birth: 22 September 1791
Place of Birth: Newington Butts, England
Date of Death: 25 August 1867 (aged 75)
Place of Death: Hampton Court, Middlesex, England
Diseases & Disabilities: Dyslexia
Father: James Faraday
Mother: Margaret Hastwell
Spouse: Sarah Barnard (m. 1821–1867)
Discoveries/Inventions: Electromagnetic Induction, Plane Of Polarization, Benzene
Awards: Royal Society Bakerian Medal, Copley Medal, Royal Medal, Albert Medal, Rumford Medal
Early Life

Michael Faraday was born on September 22, 1791, in London, England, UK. He was one of the most prolific scientists of the 19th century. A British physicist and chemist, Faraday is best known for his discoveries of electromagnetic induction and the laws of electrolysis.
Michael Faraday was born in the country village of Newington, Surrey, now a part of South London. His father was a blacksmith who had migrated from the north of England earlier in 1791 to look for work. His mother was a countrywoman of great calm and wisdom who supported her son emotionally through a difficult childhood. Faraday was one of four children, all of whom were hard put to get enough to eat since their father was often ill and incapable of working steadily.
In 1804, he served as an errand boy for the bookseller George Riebau, delivering newspapers among other things, who a year later, indentured Faraday for a period of seven years. It was during these seven years of apprenticeship that Faraday read many books, two amongst which that captured his attention like none others were Isaac Watts’, The Improvement of the Mind and Jane Marcet’s, Conversations on Chemistry. Not only did this reading activity improve his knowledge and understanding, but it also determined his course of life. Faraday’s keen interest in science, especially in electricity, was developed herein.
Although Faraday received little formal education, he was one of the most influential scientists in history. It was by his research on the magnetic field around a conductor carrying a direct current that Faraday established the basis for the concept of the electromagnetic field in physics.
Faraday also established that magnetism could affect rays of light and that there was an underlying relationship between the two phenomena. He similarly discovered the principles of electromagnetic induction and diamagnetism and the laws of electrolysis. His inventions of electromagnetic rotary devices formed the foundation of electric motor technology, and it was largely due to his efforts that electricity became practical for use in technology.
In 1820 he produced the first known compounds of carbon and chlorine, C2Cl6 and C2Cl4. These compounds were produced by substituting chlorine for hydrogen in “olefiant gas” (ethylene), the first substitution reactions induced. (Such reactions later would serve to challenge the dominant theory of chemical combination proposed by Jöns Jacob Berzelius.) In 1825, as a result of research on illuminating gases, Faraday isolated and described benzene. In the 1820s he also conducted investigations of steel alloys, helping to lay the foundations for scientific metallurgy and metallography. While completing an assignment from the Royal Society of London to improve the quality of optical glass for telescopes, he produced a glass of very high refractive index that was to lead him in 1845 to the discovery of diamagnetism. In 1821 he married Sarah Barnard, settled permanently at the Royal Institution, and began the series of researches on electricity and magnetism that were to revolutionize physics.
Faraday was a devout Christian; his Sandemanian denomination was an offshoot of the Church of Scotland. Well after his marriage, he served as a deacon and for two terms as an elder in the meeting house of his youth. His church was located at Paul’s Alley in the Barbican. This meeting house relocated in 1862 to Barnsbury Grove, Islington; this North London location was where Faraday served the final two years of his second term as elder prior to his resignation from that post. Biographers have noted that “a strong sense of the unity of God and nature pervaded Faraday’s life and work.”
Childhood, Family and Educational Life

Michael Faraday was born on 22 September 1791 in Newington Butts, which is now part of the London Borough of Southwark but was then a suburban part of Surrey. His family was not well off. His father, James was a member of the Glassite sect of Christianity. Professionally, James was an apprentice to the village blacksmith. Before marriage, his mother had been a servant. The family lived in a degree of poverty. His mother was a countrywoman of great calm and wisdom who supported her son emotionally through a difficult childhood. The young Michael Faraday, who was the third of four children, having only the most basic school education, had to educate himself.
Michael Faraday attended a local school until he was 13, where he received a basic education. To earn money for the family he started working as a delivery boy for a bookshop. He worked hard and impressed his employer. After a year, he was promoted to become an apprentice bookbinder. During his seven-year apprenticeship, Faraday read many books, including Isaac Watts’s The Improvement of the Mind, and he enthusiastically implemented the principles and suggestions contained therein. He also developed an interest in science, especially in electricity. Faraday was particularly inspired by the book Conversations on Chemistry by Jane Marcet.
In 1812, at the age of 20 and at the end of his apprenticeship, Faraday attended lectures by the eminent English chemist Humphry Davy of the Royal Institution and the Royal Society, and John Tatum, founder of the City Philosophical Society. Many of the tickets for these lectures were given to Faraday by William Dance, who was one of the founders of the Royal Philharmonic Society.
Personal Life
The wedding bells for Michael Faraday rang on June 12, 1821. His significant other, Sarah Barnard, was the daughter of the Sandemanian silversmith, Edward Barnard. The couple first met through their families at the Sandemanian church. He confessed his faith to the Sandemanian congregation the month after they were married. They had no children.
He served as a deacon and for two terms, as an elder in the meeting house of his youth. His church was located at Paul’s Alley in the Barbican. Later, in 1862, the meeting house was relocated to Barnsbury Grove, Islington which was where Faraday served the final two years of his second term as the elder before resigning from that post.
Career and Works
Faraday’s great opportunity came when he was offered a ticket to attend chemical lectures by Sir Humphry Davy at the Royal Institution of Great Britain in London. Faraday went, sat absorbed with it all, recorded the lectures in his notes, and returned to bookbinding with the seemingly unrealizable hope of entering the temple of science. He sent a bound copy of his notes to Davy along with a letter asking for employment, but there was no opening. Davy did not forget, however, and, when one of his laboratory assistants was dismissed for brawling, he offered Faraday a job. Faraday began as Davy’s laboratory assistant and learned chemistry at the elbow of one of the greatest practitioners of the day. It has been said, with some truth, that Faraday was Davy’s greatest discovery.

He was involved in the study of chlorine. Faraday also conducted experiments on the diffusion of gases. Additionally, he succeeded in liquefying several gases, investigating the alloys of steel, and producing several new kinds of glass intended for optical purposes. One of Faraday’s most notable works was an invention of the earliest form of Bunsen burner (as we call it today), which is still in use today in the science laboratories around the world as a most suitable source of heat. His extensive work in the field of chemistry can be found out from the fact that he discovered the chemical substance benzene, a chemical compound of carbon and hydrogen. Faraday also discovered two new compounds in chlorine and carbon. While one is used in smoke grenades, the other is employed in the arena of dry cleaning, and spot removing. Faraday is also credited for discovering the laws of electrolysis, and for popularizing terminology such as anode, cathode, electrode, and ion, for which he took the help of William Whewell. It is said that Faraday first reported what we today know as metallic nanoparticles. In 1847, Faraday researched that the optical properties of gold colloids differed from those of the corresponding bulk metal, and it was this discovery which marked the birth of nanoscience.
Faraday is best known for his work regarding electricity and magnetism. His first recorded experiment was the construction of a voltaic pile with seven ha’penny coins, stacked together with seven disks of sheet zinc, and six pieces of paper moistened with salt water. With this pile, he decomposed sulfate of magnesia.

In 1821, soon after the Danish physicist and chemist Hans Christian Ørsted discovered the phenomenon of electromagnetism, Davy and British scientist William Hyde Wollaston tried but failed, to design an electric motor. Faraday, having discussed the problem with the two men, went on to build two devices to produce what he called “electromagnetic rotation”. One of these, now known as the homopolar motor, caused a continuous circular motion that was engendered by the circular magnetic force around a wire that extended into a pool of mercury wherein was placed a magnet; the wire would then rotate around the magnet if supplied with current from a chemical battery.
In 1824, aged 32, he was elected to the Royal Society. This was recognition that he had become a notable scientist in his own right. In 1825, aged 33, he became Director of the Royal Institution’s Laboratory. In 1833, aged 41, he became Fullerian Professor of Chemistry at the Royal Institution of Great Britain. He held this position for the rest of his life.
In the year 1839, Faraday conducted a series of experiments to examine the fundamental nature of electricity. To produce the phenomena of electrostatic attraction, electrolysis, and magnetism, Faraday used “static”, batteries, and “animal electricity”.
In 1845, Faraday discovered that many materials exhibit a weak repulsion from a magnetic field: a phenomenon he termed diamagnetism.
Faraday also discovered that the plane of polarization of linearly polarized light can be rotated by the application of an external magnetic field aligned with the direction in which the light is moving. This is now termed the Faraday Effect. In Sept 1845 he wrote in his notebook, “I have at last succeeded in illuminating a magnetic curve or line of force and in magnetizing a ray of light”.
During his work on static electricity, Faraday’s experiment displayed that the charge resided only on the exterior of a charged conductor, and exterior charge had no influence on anything enclosed within a conductor. This was due to the fact that the exterior charges redistributed in such a way that the interior fields due to them canceled. This protective effect is used in what we now know as a Faraday cage.
Later on in his life, in 1862, Faraday used a spectroscope to search for a different alteration of light, the change of spectral lines by an applied magnetic field. The equipment available to him was, however, insufficient for a definite determination of spectral change. Pieter Zeeman later used an improved apparatus to study the same phenomenon, publishing his results in 1897 and receiving the 1902 Nobel Prize in Physics for his success. In both his 1897 paper and his Nobel acceptance speech, Zeeman made reference to Faraday’s work.
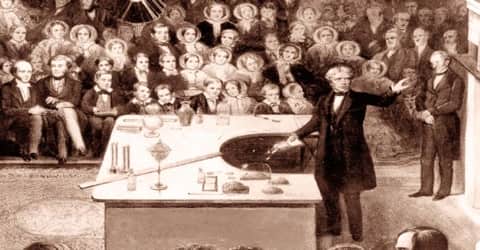
Faraday is known to have given Christmas lectures for a record nineteen times between 1827 and 1860. For this accomplishment, the University of Oxford granted Faraday a Doctor of Civil Law degree (honorary) in June 1832. In1838, he was elected a foreign member of the Royal Swedish Academy of Sciences and later in 1844, Faraday became one of eight foreign members elected to the French Academy of Sciences. Meanwhile, in his life, Faraday declined the offer of a knighthood and twice refused the post of the President of the Royal Society which was offered to him. In 1848, Michael Faraday was honored a grace and favor house in Hampton Court in Middlesex, free of all expenses or upkeep, as a result of representations by the Prince Consort. Ten years later, he retired and lived there.

In 1848, aged 54, and again in 1858 he was offered the Presidency of the Royal Society, but he turned it down.
Over the course of several letters to his close friend Benjamin Abbott, Faraday outlined his recommendations on the art of lecturing: Faraday wrote: “a flame should be lighted at the commencement and kept alive with unremitting splendor to the end”. His lectures were joyful and juvenile, he delighted in filling soap bubbles with various gasses (in order to determine whether or not they are magnetic) in front of his audiences and marveled at the rich colors of polarized lights, but the lectures were also deeply philosophical. In his lectures he urged his audiences to consider the mechanics of his experiments: “you know very well that ice floats upon water … Why does the ice float? Think of that, and philosophise”. His subjects consisted of Chemistry and Electricity, and included: 1841 The Rudiments of Chemistry, 1843 First Principles of Electricity, 1848 The Chemical History of a Candle, 1851 Attractive Forces, 1853 Voltaic Electricity, 1854 The Chemistry of Combustion, 1855 The Distinctive Properties of the Common Metals, 1857 Static Electricity, 1858 The Metallic Properties, 1859 The Various Forces of Matter and their Relations to Each Other.
Honors
In honor and remembrance of his great scientific contributions, several institutions have created prizes and awards in his name. This include:
- The IET Faraday Medal
- The Royal Society of London Michael Faraday Prize
- The Institute of Physics Michael Faraday Medal and Prize
- The Royal Society of Chemistry Faraday Lectureship Prize
Death and Legacy

Michael Faraday breathed his last on August 25, 1867, at his house at Hampton Court. He was buried in the dissenters’ (non-Anglican) section of Highgate Cemetery, after turning down the burial in Westminster Abbey. Nevertheless, Faraday has a memorial plaque near Newton’s tomb. In order to pay tribute to the works of this great scientist, a statue of Faraday stands in Savoy Place, London, outside the Institution of Engineering and Technology. London also houses a memorial in the memory of Faraday, which is situated at the Elephant & Castle gyratory system, near Faraday’s birthplace at Newington Butts. Designed by brutalist architect Rodney Gordon, the memorial commemorates Michael Faraday’s importance as a scientist. Walworth, London not only has a small park by the name Faraday Gardens, but also a school which is known as Michael Faraday Primary school. Located on Trinity Buoy Wharf is the Faraday School, where his workshop stands until today above the Chain and Buoy Store, alongside London’s only lighthouse.

Streets named for Faraday can be found in many British cities (e.g., London, Fife, Swindon, Basingstoke, Nottingham, Whitby, Kirkby, Crawley, Newbury, Swansea, Aylesbury and Stevenage) as well as in France (Paris), Germany (Berlin-Dahlem, Hermsdorf), Canada (Quebec; Deep River, Ontario; Ottawa, Ontario), and the United States (Reston, Virginia).

A Royal Society of Arts blue plaque, unveiled in 1876, commemorates Faraday at 48 Blandford Street in London’s Marylebone district. From 1991 until 2001, Faraday’s picture featured on the reverse of Series E £20 banknotes issued by the Bank of England. He was portrayed conducting a lecture at the Royal Institution with the magneto-electric spark apparatus. In 2002, Faraday was ranked number 22 in the BBC’s list of the 100 Greatest Britons following a UK-wide vote.
Information Source: